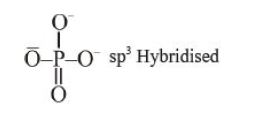A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hybridisation of 'P' in PO4^(3-) is same as that of : -
Text Solution
|
- The hybridisation of P in phosphate ion (PO4^(2-)) is the same as in :
Text Solution
|
- Hybridisation of 'P' in PO4^(3-) is same as that of : -
Text Solution
|
- Hybridisation of 'P' in PO4^(3-) is same as that of : -
Text Solution
|
- Oxidation number of P in PO4^(3-) ion is :
Text Solution
|
- Oxidation state of P in PO4^(3-) is
Text Solution
|
- Oxidation state of P in PO4^(3-) is
Text Solution
|
- Oxidation state of P in PO4^(3-) is
Text Solution
|
- Oxidation state of P in PO4^(3-) is
Text Solution
|