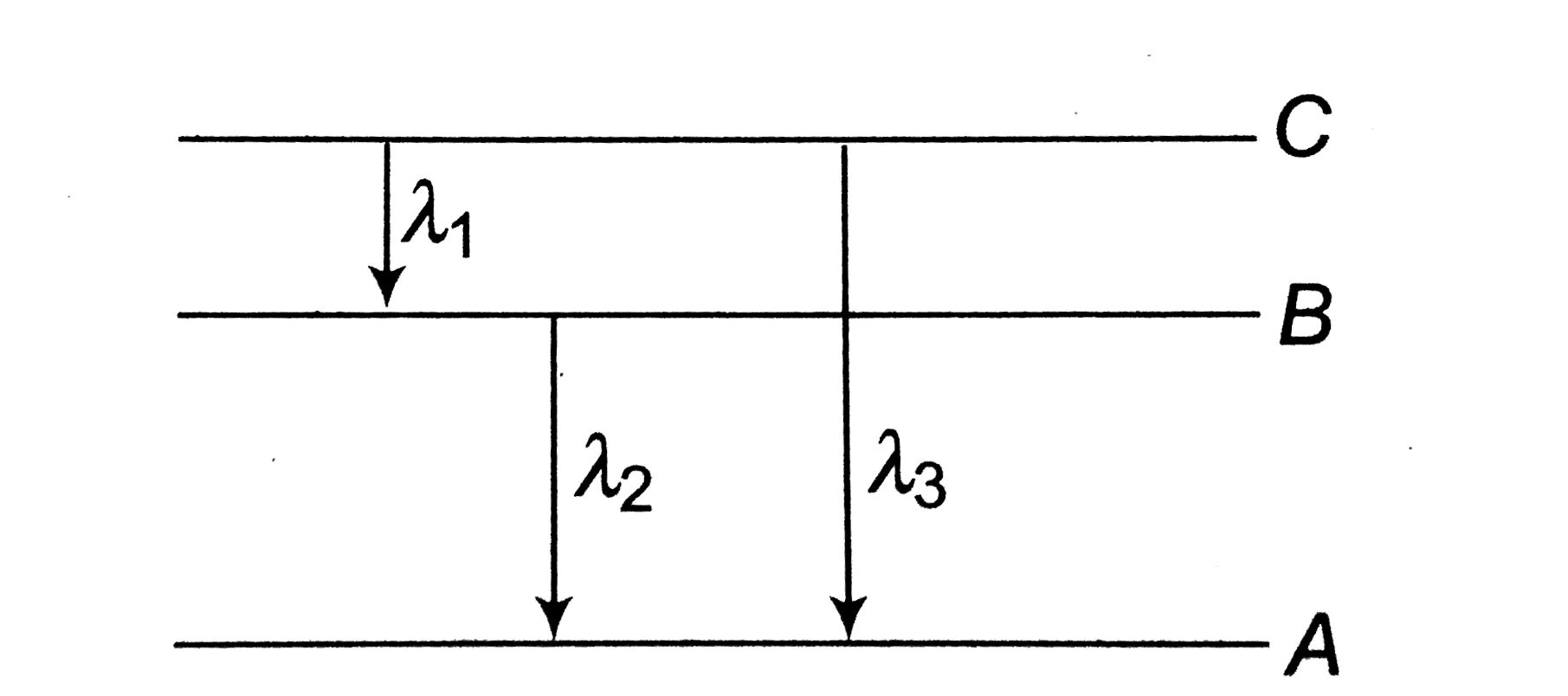
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-ATOMIC PHYSICS-Atomic Physics
- The Bohr model of atoms
Text Solution
|
- Which of the following transitions in a hydrogen atom emits photon of ...
Text Solution
|
- When electron jumps from n = 4 to n = 1 orbit, we get
Text Solution
|
- In the Bohr model of a hydrogen atom, the centripetal force is furnish...
Text Solution
|
- The groud state energy of hydrogen atom is -13.6 eV. When its electron...
Text Solution
|
- When hydrogen atom is in first excited level, its radius is….its groun...
Text Solution
|
- when a hydrogen atom is raised from the ground state to an excited sta...
Text Solution
|
- The spectrum obtained from a sodium vapour lamp is an example of
Text Solution
|
- The radius of hydrogen atom in its ground state is 5.3 xx 10^(-11)m. A...
Text Solution
|
- In Rutherford scattering experiment, what will b ethe correct angle fo...
Text Solution
|
- Hydrogen atoms are excited from ground state of the principle quantum ...
Text Solution
|
- Which source is associated with a line emission spectrum?
Text Solution
|
- The ionisation energy of hydrogen atom is 13.6 eV. Following Bohr's th...
Text Solution
|
- In terms of Bohr radius a(0), the radius of the second Bohr orbit of a...
Text Solution
|
- Ground state energy of H-atom is -13.6 eV. The energy needed to ionise...
Text Solution
|
- Consider an eelctron in the nth orbit of a hydrogen atom in the Bohr m...
Text Solution
|
- The valence electron in alkali metal is a
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- To explain his theory, Bohr used
Text Solution
|
- The ionization energy of hydrogen atom is 13.6 eV. Calculate Rydberg's...
Text Solution
|