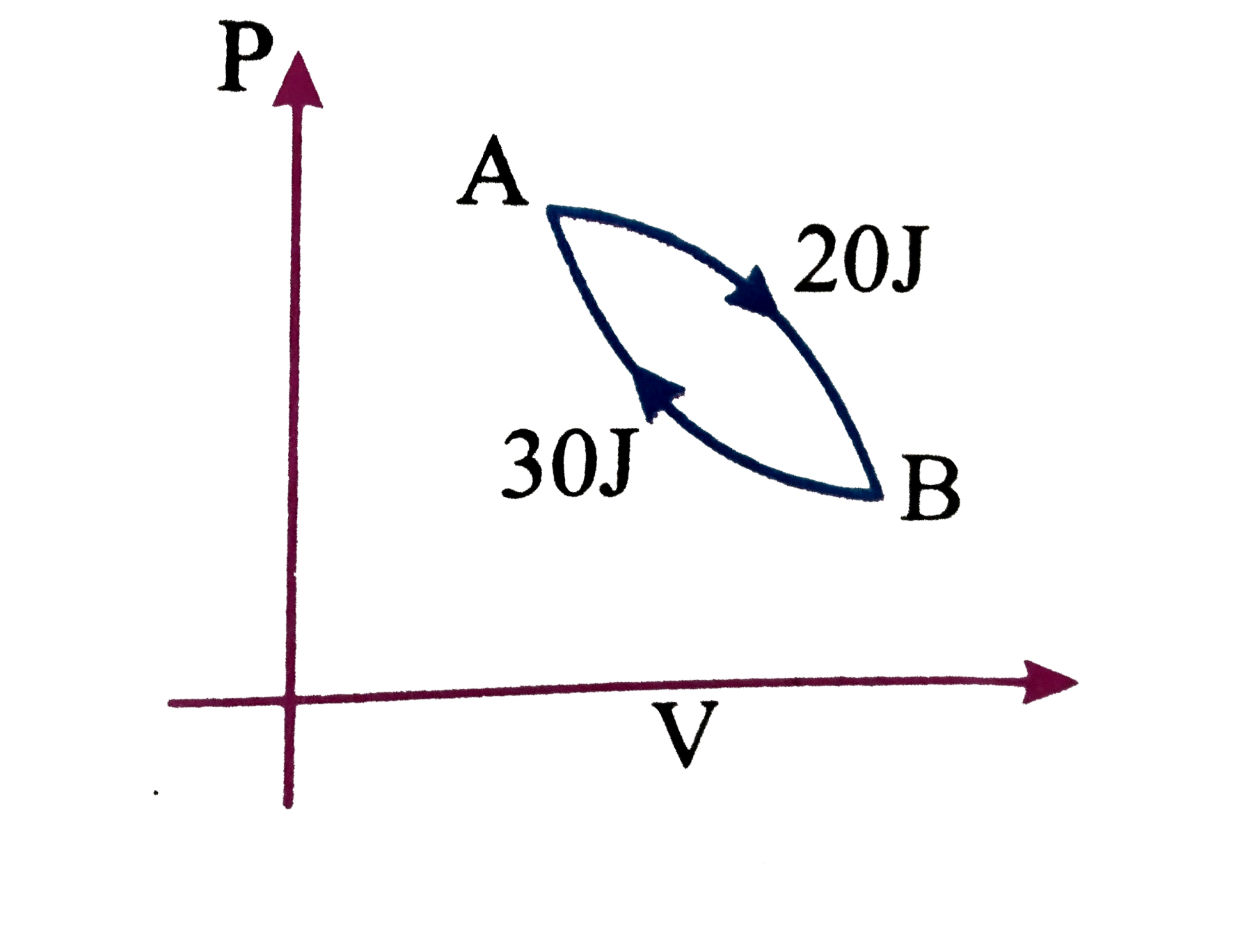
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a cyclic process shown in the figure an ideal gas is adial gas is a...
Text Solution
|
- An ideal gas is taken round a cyclci thermodynamic process ABCA as sho...
Text Solution
|
- A certain mass of is taken from an initial thermodynamics state A to a...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adial gas is a...
Text Solution
|
- An ideal gas follows a cyclic process as shown in figure. Internal ene...
Text Solution
|
- An ideal gas is taken in a cyclic process as shown in the figure. Calc...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adiabatically ...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA , as shown in the f...
Text Solution
|
- In a cyclic process shown in the figure on ideal gas is adiabatically ...
Text Solution
|