Similar Questions
Explore conceptually related problems
Recommended Questions
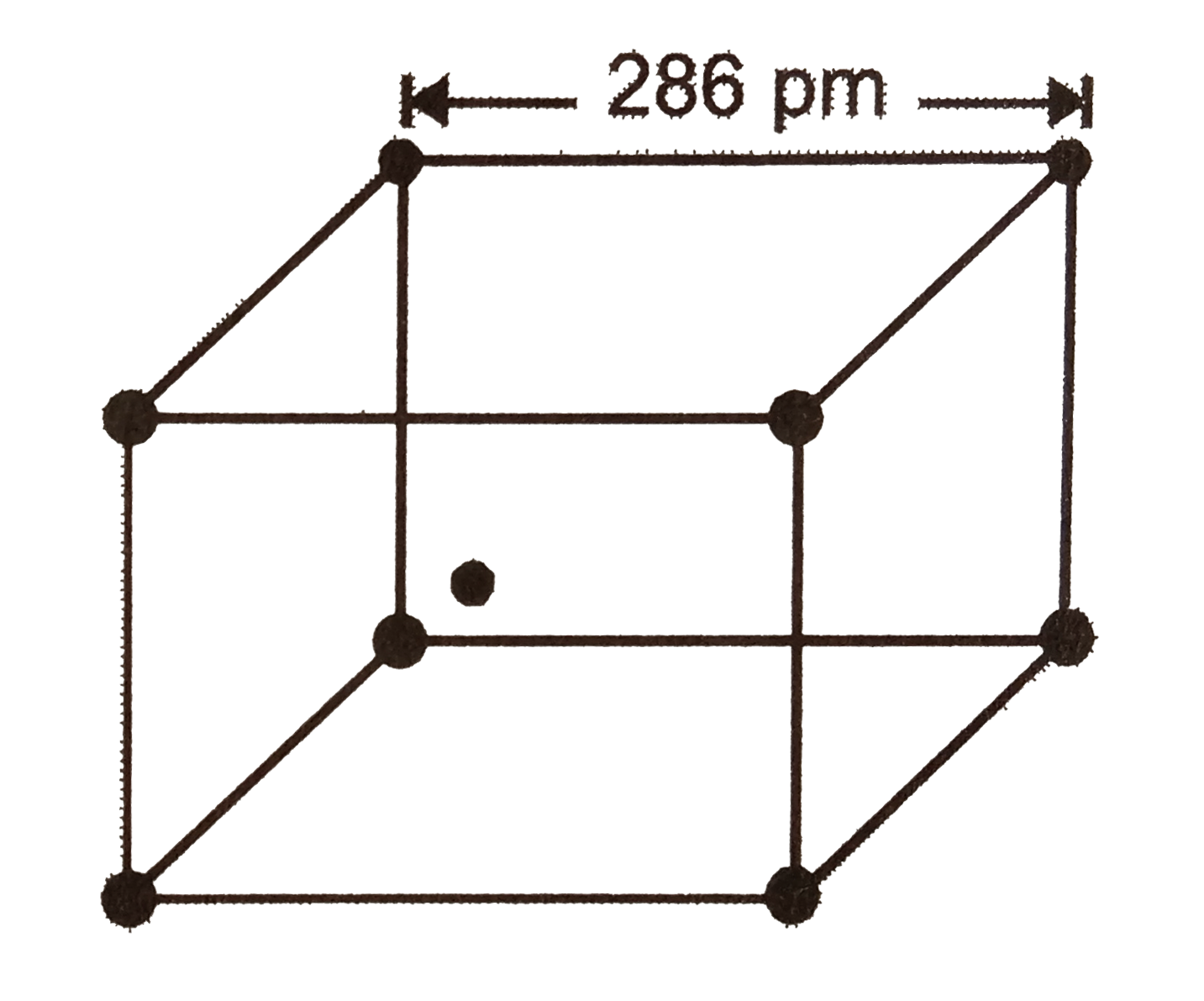
- The crystal structure adopted by iron is shown below. The distance bet...
Text Solution
|
- The crystal structure adopted by iron is shown below. The distance bet...
Text Solution
|
- The mean distance between the atoms of iron is 3xx10^(-10) m and inter...
Text Solution
|
- Iron has body centred cubic lattice. If the edge length of the unit ce...
Text Solution
|
- Iron crystalizes in two bcc lattices, the alpha- from below 910^(@) an...
Text Solution
|
- कमरे के तापमान पर लोहा अन्तःकेन्द्रित घनीय संरचना में क्रिस्टलित होता ...
Text Solution
|
- लोहा अन्तः केंद्रित तथा फलक केंद्रित घनीय के रुप में स्थित हो सकता है ...
Text Solution
|
- आयरन अंतः केन्द्रित घनीय जालक के रूप में क्रिस्टलीकृत होता है। यदि इक...
Text Solution
|
- Iron crystallizes in several modifications. At about 910^(@)C , the bo...
Text Solution
|