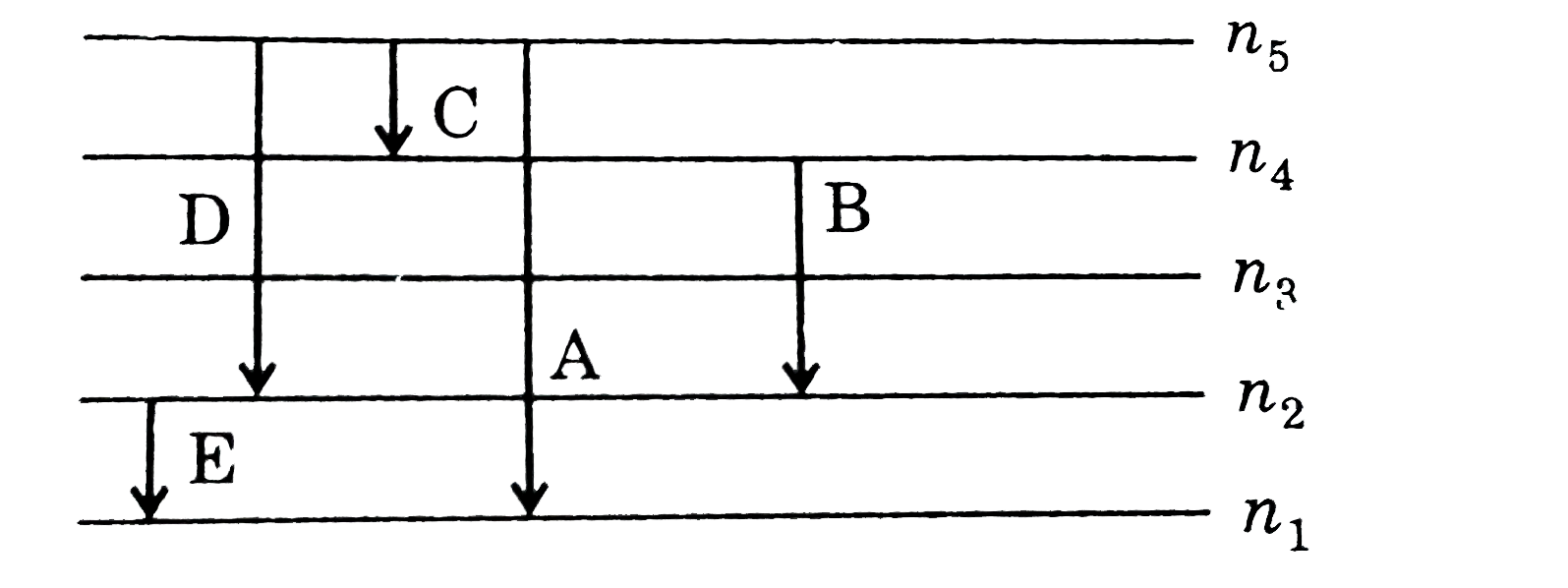
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ATOMIC STRUCTURE-Multiple Objective Type
- For two Bohr orbits n(1) "and"n(2) which follow the relationships, (n(...
Text Solution
|
- Select the correct statement about electromagnetic waves.
Text Solution
|
- Whenever a hydrogen atom emits photon in the Balmer series:
Text Solution
|
- Radiatonal wavelength (lambda)=124nm falls on a metallic surface then ...
Text Solution
|
- For a H-like atom which Bohr's model some spectral lines were observed...
Text Solution
|
- Select the correct statement .
Text Solution
|
- Choose the correct statements regarding 'Psi'.
Text Solution
|
- (i) A plot of 4pir^(2) R^(2) vs r has a total three maximas. (ii) Th...
Text Solution
|
- The orbit angular momentum of 3d and 4d are:
Text Solution
|
- Identify the correct statement (s) regarding 3p(z) orbital.
Text Solution
|
- Maximum probability region (r(max) "values") for an orbital increases ...
Text Solution
|
- If r(max) represents the longest maximum probability region and ltr(ma...
Text Solution
|
- Select the correct statement (s)
Text Solution
|
- Select correct statement(s).
Text Solution
|
- The wave function for 2s orbital is given as: Psi = ((1)/(sqrt2)) ((...
Text Solution
|
- A hydrogen like atom in ground state absorbs n photon having the sa...
Text Solution
|
- Choose the correct statement among the following
Text Solution
|
- Correct statement (s) regarging 3p(y) orbital is//are :
Text Solution
|
- Choose the incorrect statement (s) :
Text Solution
|
- Which of the following is//are correct statement (s).
Text Solution
|