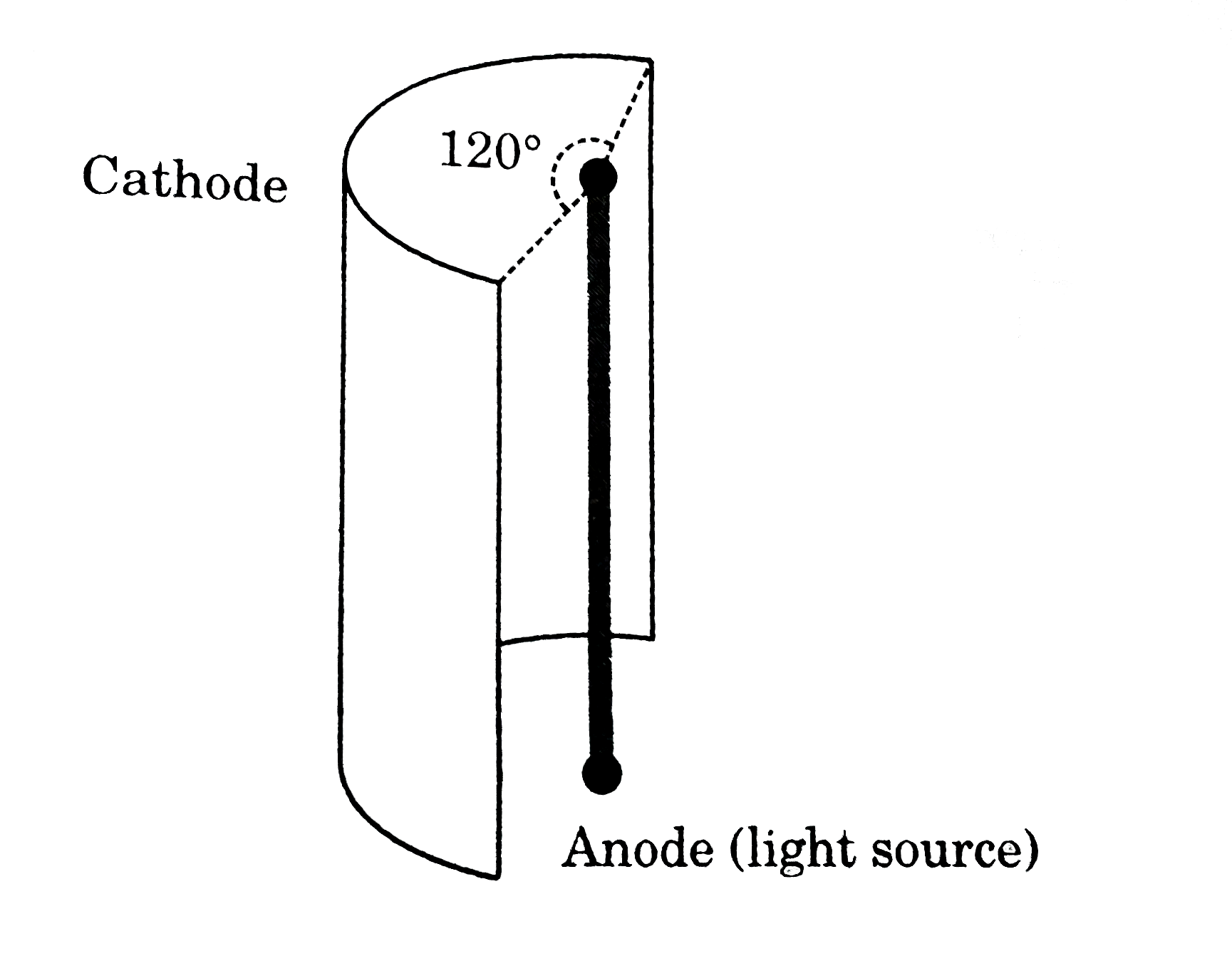Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ATOMIC STRUCTURE-Subjective Type
- Total different spectral lines observed in between 11th excited state ...
Text Solution
|
- On a metal with work function 2.0 eV light of wavelength 400 nm falls,...
Text Solution
|
- By a sample of ground state atomic hydrogen ,UV light of energy (13.6x...
Text Solution
|
- 'alpha particle' of 3.6 MeV are fired toward nucleus .(Z)^(A)X, at poi...
Text Solution
|
- Find the number of spectral lines in Paschen series emitted by atomic ...
Text Solution
|
- Calculate the minimum kinetic energy in eV of photoelectron produced...
Text Solution
|
- A beam of light has three lambda, 4144 "Å", 4972 "Å" "and"6216 "Å" wit...
Text Solution
|
- A particle of charge equal to that of electron and mass 208 times the ...
Text Solution
|
- Calculate the wavelength of a rested electron (in "Å") after it absorb...
Text Solution
|
- A hydrogen like species with atomic number 'Z' is in higher excited st...
Text Solution
|
- barr, average distance also described as expectation value of the dis...
Text Solution
|
- The number of lines of Balmer series of H-atom that belong to visible ...
Text Solution
|
- In a sample of three H-atoms , all in the 5th excited state, electrons...
Text Solution
|
- If a metal is exposed with light of wavelength lambda, the maximum kin...
Text Solution
|
- If n(1)+n(2) = 4 "and" n(2)^(2)-n(1)^(2)= 8, then calculate maximum va...
Text Solution
|
- Suppose the potential energy between electron and proton at a distance...
Text Solution
|
- A cylindrical source of light which emits radiation radially (from cur...
Text Solution
|
- Calculate the energy (in KJ) required to excite one litre of hydrogen ...
Text Solution
|
- The work function (phi) of some metals is listed below . The number of...
Text Solution
|
- The atomic masses of He and Ne are 4 and 20 amu respectively . The va...
Text Solution
|