A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GASEOUS STATE-Exercise
- A motorist inflates the tyres of her car to a pressure of 180 kPa on a...
Text Solution
|
- O(2) gas is placed in a 4 litre container containing 3 L of liquid wat...
Text Solution
|
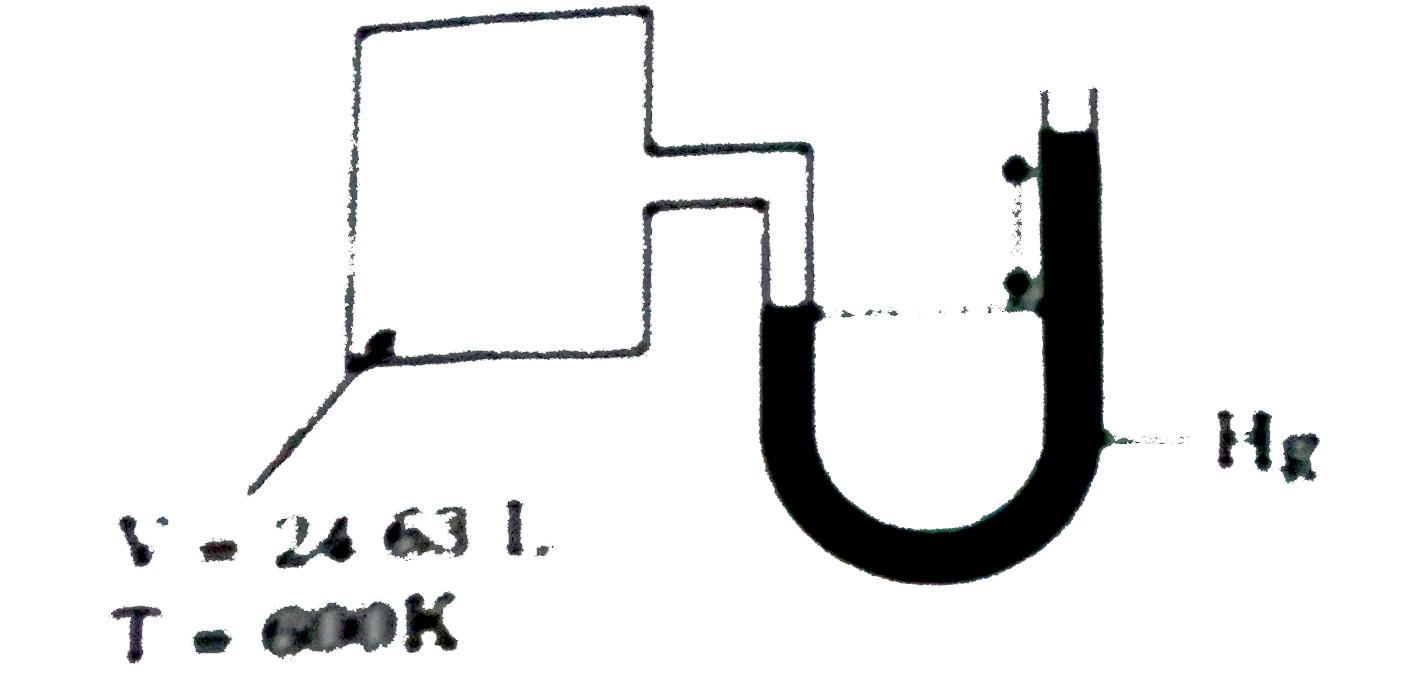
- From the following set-up, calculate moles of the gas present in the c...
Text Solution
|
- If air is assumed to be at temperature 290K with molar mass 29 gm/mol ...
Text Solution
|
- On the surface of the earth at 1 atm pressure, a balloon filled with H...
Text Solution
|
- A quantity of gas is collected in a graduated tube over the mercury. T...
Text Solution
|
- A gas A(x) having mass of 100g is confined in a container of volume 16...
Text Solution
|
- In a glass tube columns of water and mercury appear as shown. Thi...
Text Solution
|
- Moist air is less dense than dry air at the same temperature and barom...
Text Solution
|
- What is the major reason for using mercury (rather than water) in baro...
Text Solution
|
- A gas is collected in the flask shown here. What is the pressure exert...
Text Solution
|
- The vapour pressure of water at 20^(@)C is 17.54 mmHg. What will be th...
Text Solution
|
- At 20^(@)C vapour pressure of H(2)O(l) is recorded as 28.57m bar. The ...
Text Solution
|
- A closed end manometer is filled with Hg as shown in the following dia...
Text Solution
|
- At any top of the mountain the thermoneterreads 0^(@)C and the haromet...
Text Solution
|
- A certain mountain is 14.100 feet above sea-level. The pressure a the ...
Text Solution
|
- Find pressure (in atm) at point A, 10 cm above the bottom of container...
Text Solution
|
- A contaienr of volume 2 litre contains H(2) gas at 300 K as shown (P("...
Text Solution
|
- In the figure shown the pressure of the confined gas will be:
Text Solution
|
- Total pressure at point (X) is:
Text Solution
|