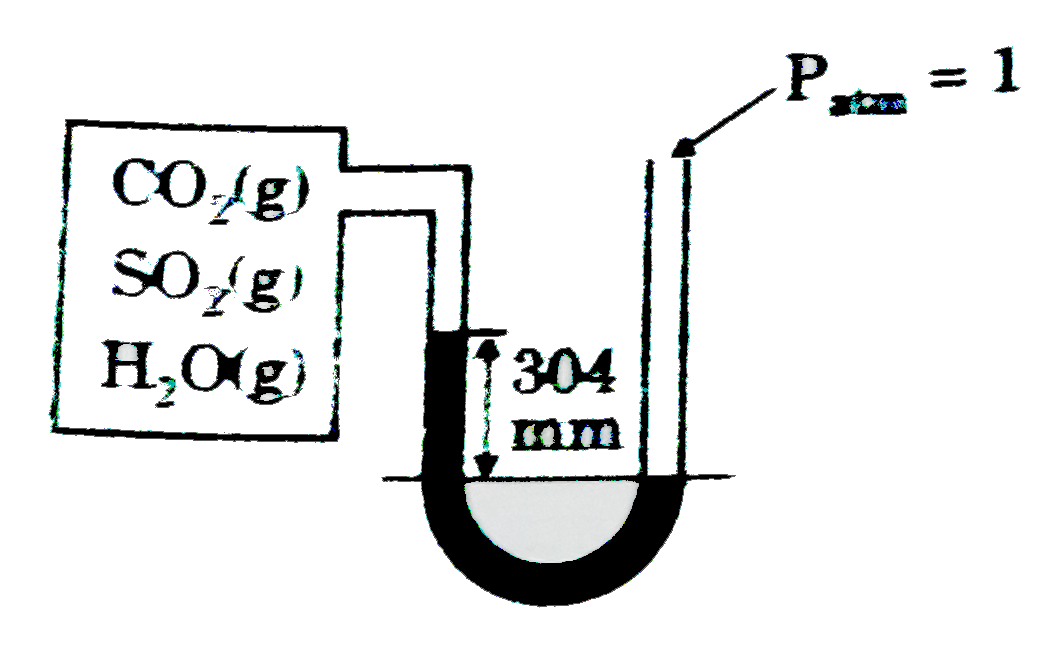A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
GRB PUBLICATION-GASEOUS STATE-Exercise
- Which of the following statement(s) is/are correct?
Text Solution
|
- For one mole of any van der Waal's gas a =0.27 atm-"litre"^(2)//"mol"^...
Text Solution
|
- A 82.1 L container connected with manometer contains mixture of CS(2) ...
Text Solution
|
- For a real gas, following equation (P+(a)/(TV(m)^(2)))(V(m)-beta)=RT, ...
Text Solution
|
- Two bulbs of volumes 200 cm^(3) and 100 cm^(3) are connected by a shor...
Text Solution
|
- van der Waal's equation of state for real gases may be written as: P...
Text Solution
|
- For O(2)(g) at 1 atm and 300K is performing 4xx10^(9) collisions per s...
Text Solution
|
- If Cl(2) and PCl(3) can react to form PCl(5) according to following re...
Text Solution
|
- Choose the correct statement(s) among the following.
Text Solution
|
- Choose the correct alternative (more than one may be correct)(B.M.C.=B...
Text Solution
|
- Select the correct option.
Text Solution
|
- A closed vessel at temperature T contains a mixture of two diatomic ga...
Text Solution
|
- Select the correct option(s) for an ideal gas.
Text Solution
|
- According to kinetic theory of gases, for a diatomic molecule wich is ...
Text Solution
|
- Choose the incorrect statement.
Text Solution
|
- Which of the following are correct for real gases?
Text Solution
|
- 5 moles gas are introduced in 1 litre container at 47^(@)C. Select the...
Text Solution
|
- 1 mol N(2) and 3 mol H(2) are introduced in 8.21 litre container initi...
Text Solution
|
- Select the correct option(s)
Text Solution
|
- A container fitted with frictionless massless piston consists of five ...
Text Solution
|