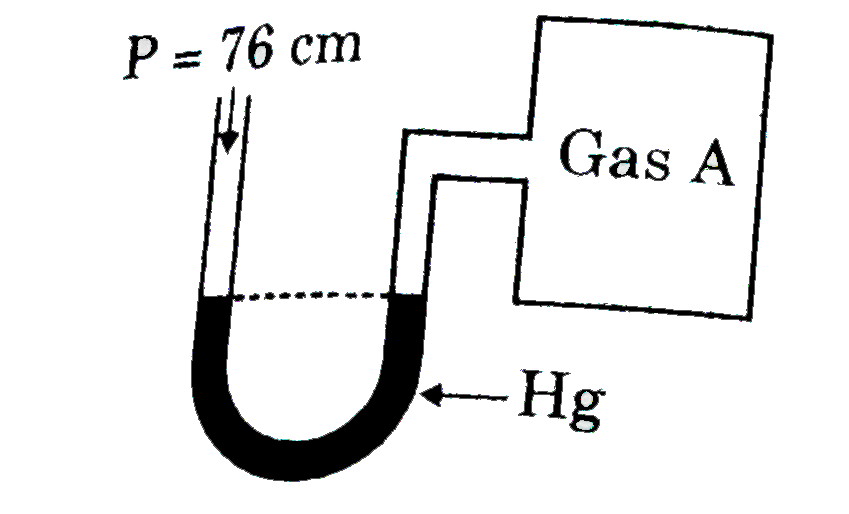A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GASEOUS STATE-Exercise
- Select the correct option(s)
Text Solution
|
- A container fitted with frictionless massless piston consists of five ...
Text Solution
|
- An open ended mercury mnometer is used to measure the pressure exerted...
Text Solution
|
- According to kinetic theory of gases:
Text Solution
|
- Choose the correct statement(s) for real gases.
Text Solution
|
- Which of the assumptions/postulates of kinetics theory does not hold g...
Text Solution
|
- If distance between 2 molecuels is r then lim (1)/(r^(x)) will result ...
Text Solution
|
- Choose the correct statement(s) regarding Boyle's point.
Text Solution
|
- According to ideal gas equation (PV)/(nT) for ga will always be consta...
Text Solution
|
- According to ideal gas equation (PV)/(nT) for ga will always be consta...
Text Solution
|
- According to ideal gas equation (PV)/(nT) for ga will always be consta...
Text Solution
|
- If same amount of gas is trapped over liqquid (a) and liquid (b) in fo...
Text Solution
|
- If same amount of gas is trapped over liquid (a) and liquid (b) in fol...
Text Solution
|
- If same amount of gas is trapped over liquid (a) and liquid (b) in fol...
Text Solution
|
- Equal masses (W gram each) of three non- reacting gases X,Y and Z were...
Text Solution
|
- Equal masses (W gram each) of three non- reacting gases X,Y and Z were...
Text Solution
|
- Equal masses (W gram each) of three non- reacting gases X,Y and Z were...
Text Solution
|
- The constant motion and high velocities of gas particles lead to some ...
Text Solution
|
- The constant motion and high velocities of gas particles lead to some ...
Text Solution
|
- The constant motion and high velocities of gas particles lead to some ...
Text Solution
|