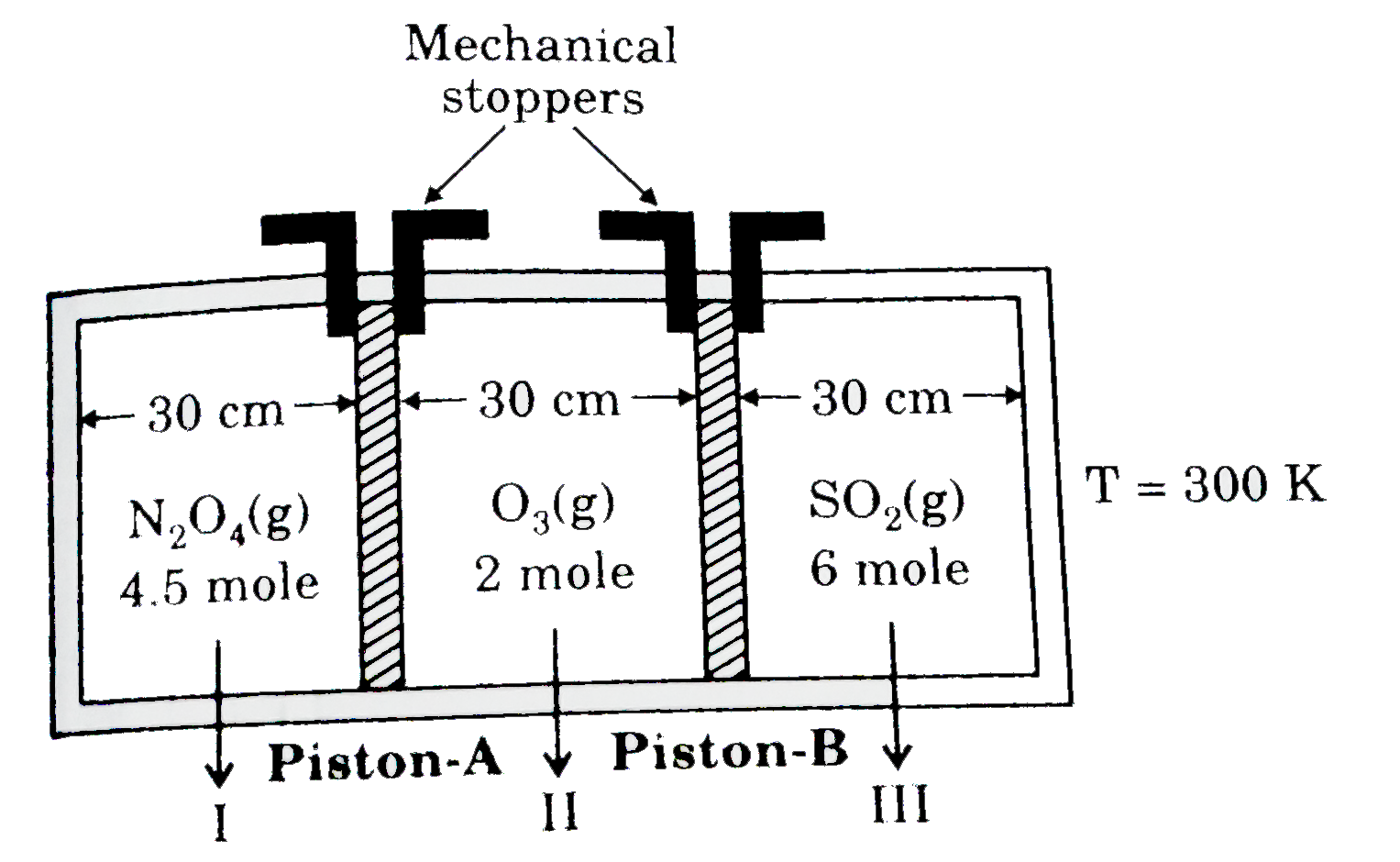
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GASEOUS STATE-Exercise
- A container is divided into two compartments. One compartment contains...
Text Solution
|
- The figure shows initial conditions of a uniform cylinder with frictio...
Text Solution
|
- The figure shows initial conditions of a uniform cylinder with frictio...
Text Solution
|
- A gaseous mixture comprising of equal moles of H(2)//O(2)//M (M mass=1...
Text Solution
|
- A gaseous mixture comprising of equal moles of H(2)//O(2)//M (M mass=1...
Text Solution
|
- Nitric oxide (NO) reacts with molecular oxygen as follows: 2NO(g)+O(...
Text Solution
|
- Nitric oxide (NO) reacts with molecular oxygen as follows: 2NO(g)+O(...
Text Solution
|
- Nitric oxide (NO) reacts with molecular oxygen as follows: 2NO(g)+O(...
Text Solution
|
- Under a given condition, it is found that two separate gases effuse ou...
Text Solution
|
- Under a given condition, it is found that two separate gases effuse ou...
Text Solution
|
- For the data IF temperature is decreased from a very high value...
Text Solution
|
- For the data For which gas is Z expected to be gt 1 always?
Text Solution
|
- For the data If adsorption of gases is done on a charcoal surface wh...
Text Solution
|
- A barometer is an instrument that is used for the measurement of press...
Text Solution
|
- A barometer is an instrument that is used for the measurement of press...
Text Solution
|
- A barometer is an instrument that is used for the measurement of press...
Text Solution
|
- {:(,"Column-I",,"Column-II"),("(a)","For a gas repulsive tendency domi...
Text Solution
|
- Match the following Column-I to Column-II
Text Solution
|
- {:(,"Coloumn-I",,"Column-II"),("(a)","At low pressure",,(p)Z=1+(pb)/(R...
Text Solution
|
- Match gases under specified conditions listed in Coloum-I with their p...
Text Solution
|