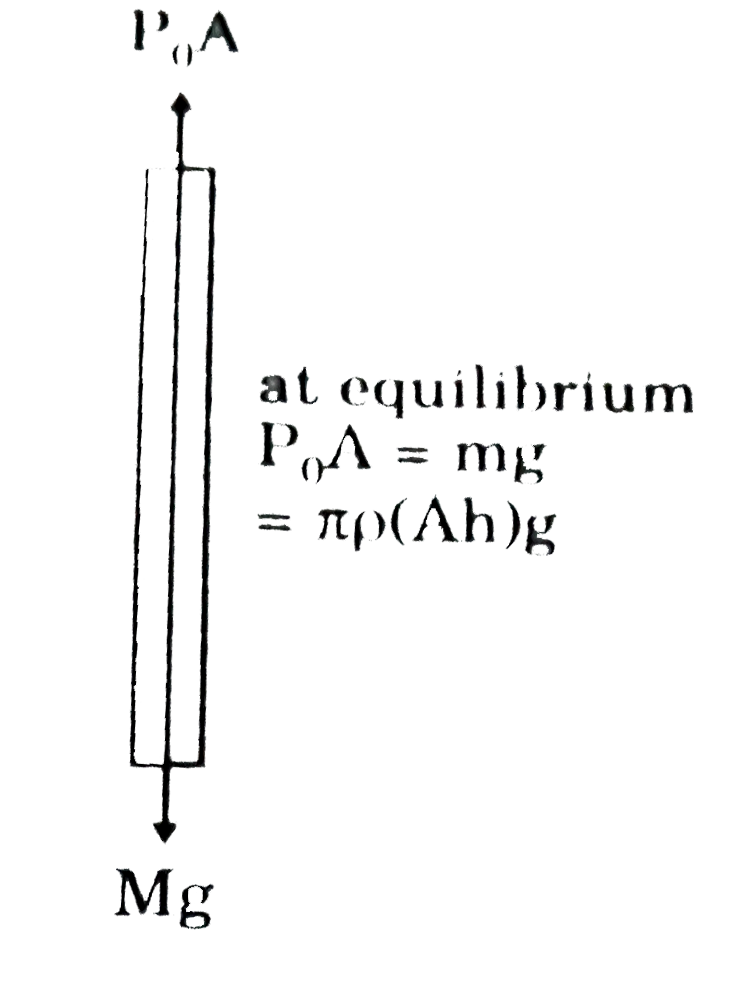A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
GRB PUBLICATION-GASEOUS STATE-Exercise
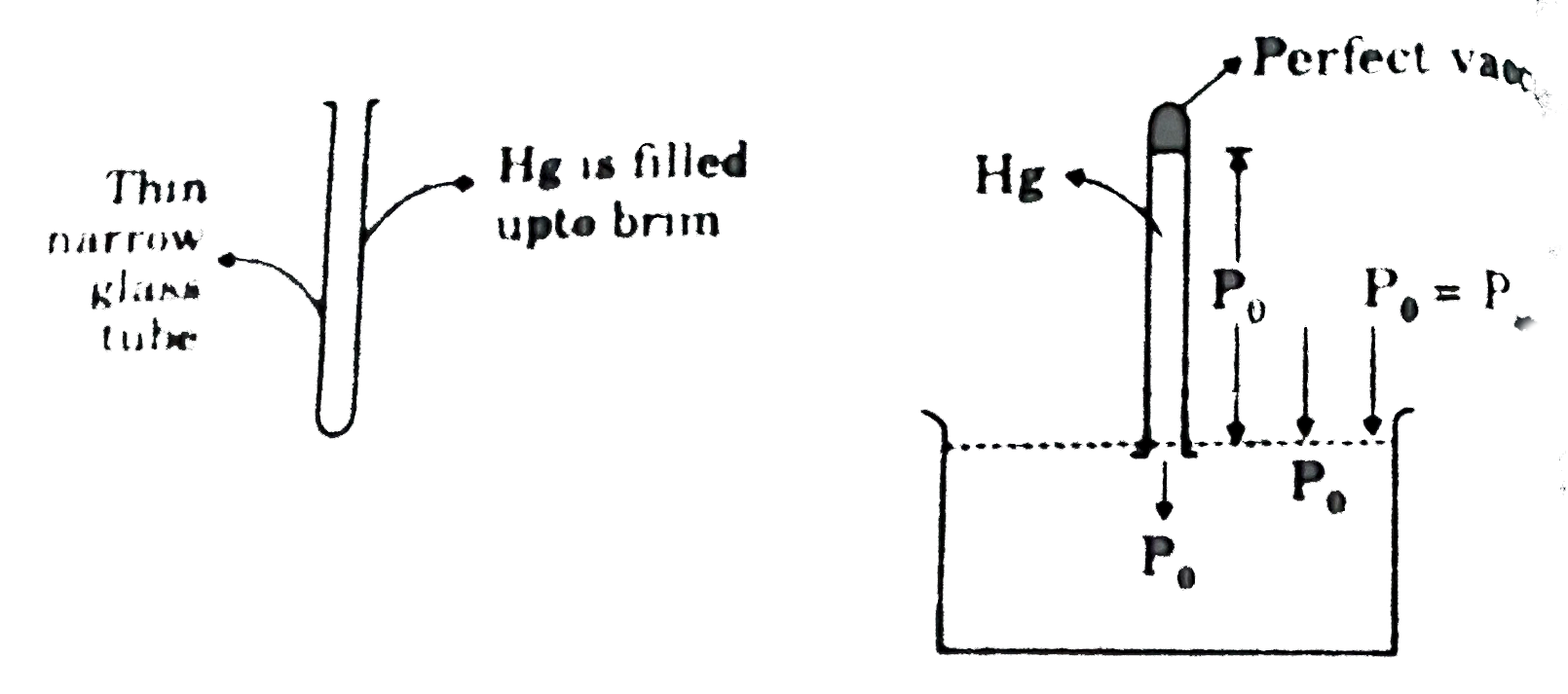
- A barometer is an instrument that is used for the measurement of press...
Text Solution
|
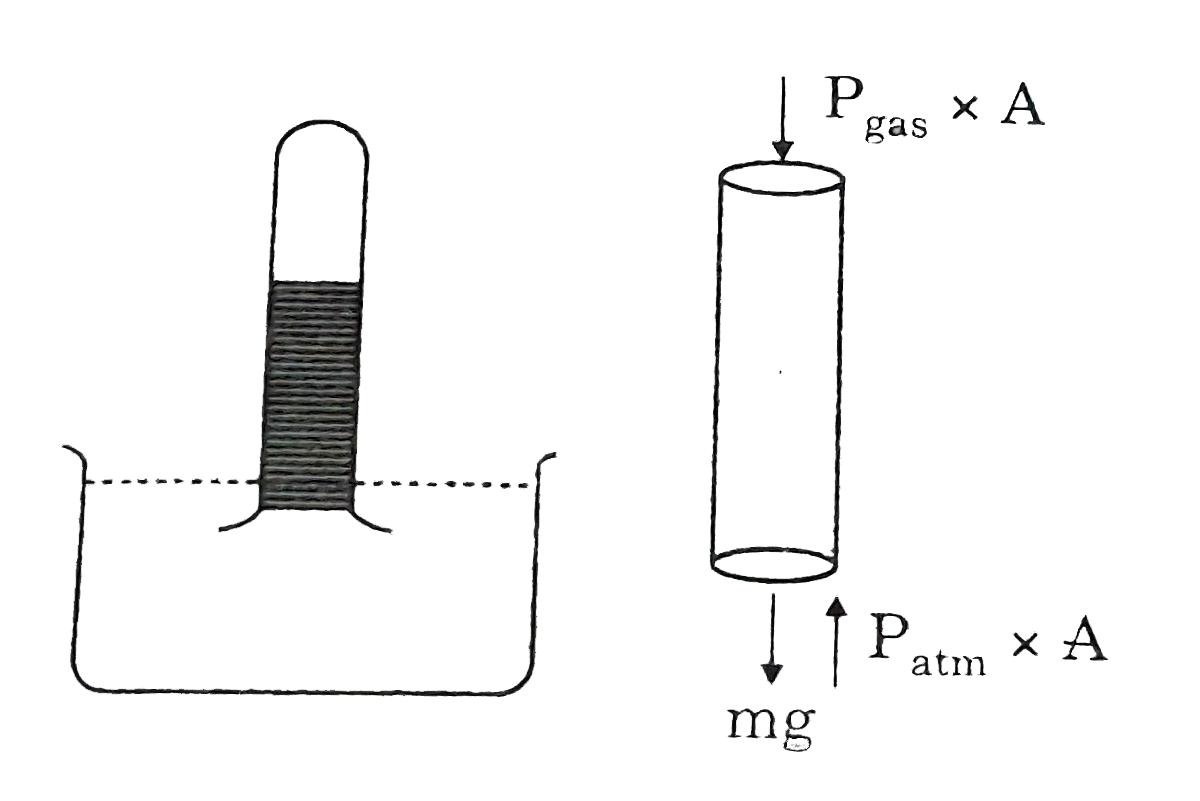
- A barometer is an instrument that is used for the measurement of press...
Text Solution
|
- A barometer is an instrument that is used for the measurement of press...
Text Solution
|
- {:(,"Column-I",,"Column-II"),("(a)","For a gas repulsive tendency domi...
Text Solution
|
- Match the following Column-I to Column-II
Text Solution
|
- {:(,"Coloumn-I",,"Column-II"),("(a)","At low pressure",,(p)Z=1+(pb)/(R...
Text Solution
|
- Match gases under specified conditions listed in Coloum-I with their p...
Text Solution
|
- Match of following (where U(rms)=root mean square speed U(av) =average...
Text Solution
|
- {:(,"List-I(Van der Waals equation)",,"List-II(given by)"),("(a)","hig...
Text Solution
|
- One mole of N(2) (g) is taken in 1 litre empty container fitted with a...
Text Solution
|
- Column-II gives the values of van der Waal's constant 'a' of a few gas...
Text Solution
|
- {:(,"Column-I",,"Column-II"),("(a)","Gas at critical temperature",,"(p...
Text Solution
|
- Sample of different gases are given at different conditions in column-...
Text Solution
|
- {:(,"Column-I",,"Column-II"),("(P)","If volume of gas molecules be neg...
Text Solution
|
- 6 litres of H(2)O is placed in a closed room of volume 827 litre and t...
Text Solution
|
- If two moles of an ideal gas at 546 K occupies a volume of 44.8 litres...
Text Solution
|
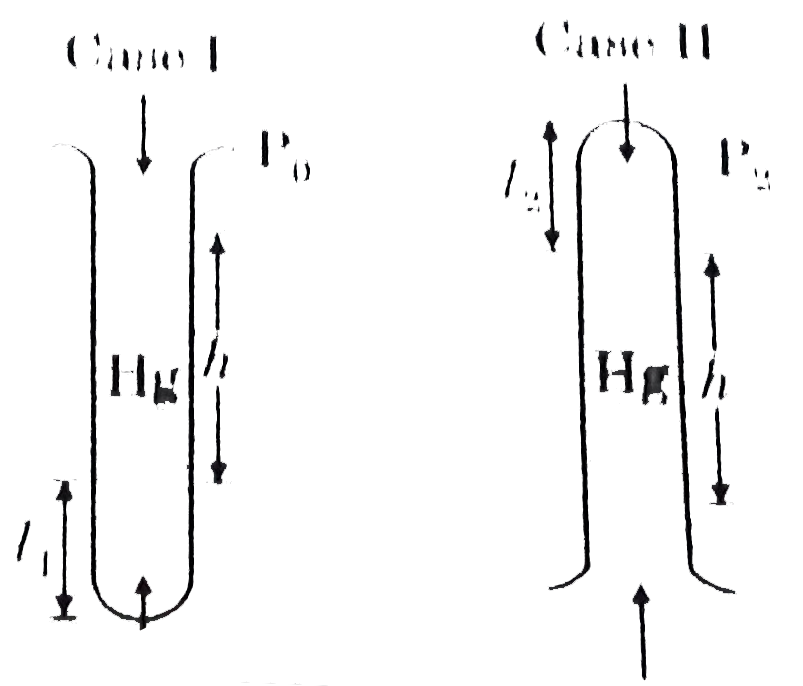
- A glass tube with a sealed end is completely submerged in a vessel wit...
Text Solution
|
- An ideal gas is trapped between a mercury column and the closed lower ...
Text Solution
|
- Two gases A and B having molecular weight 60 and 45 respectively are e...
Text Solution
|
- Pressure in a bulb dropped from 2000 to 1500 mm in 50 minuite when the...
Text Solution
|