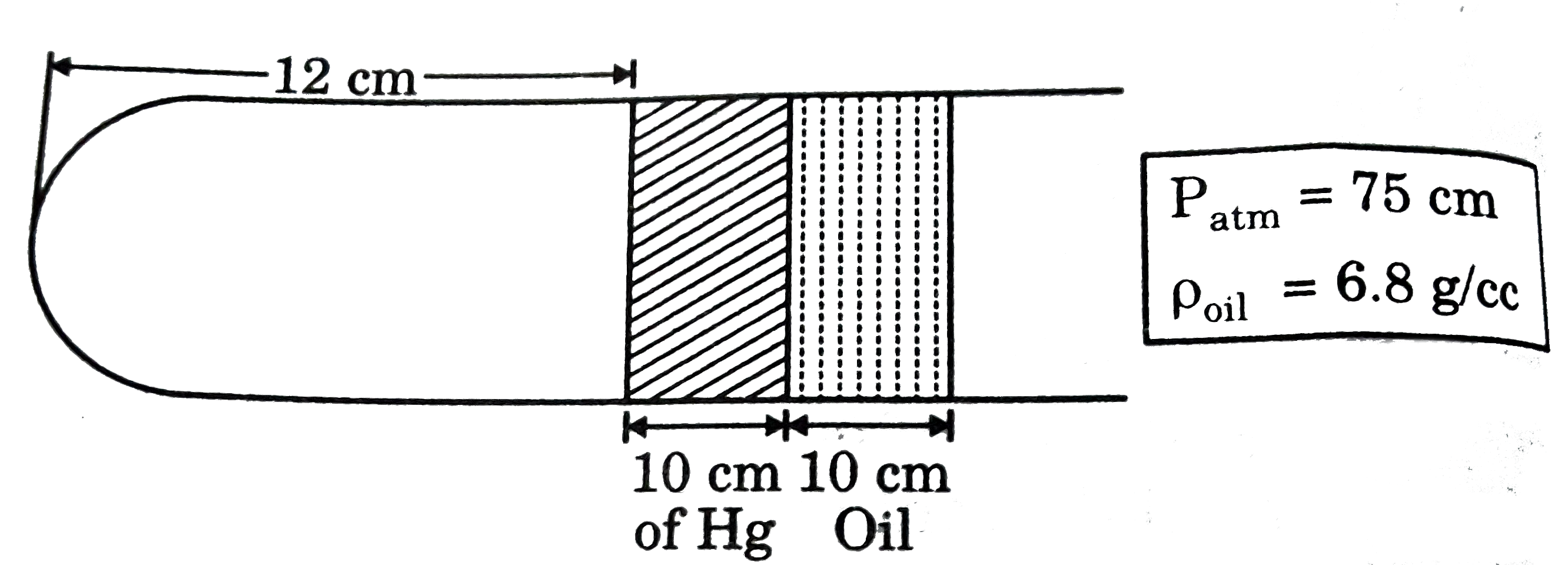
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GASEOUS STATE-Exercise
- At 300 K, two gases are filled in two equal sized containers as given ...
Text Solution
|
- A thin glass tube (uniform cross-section) is filled with He and SO(2) ...
Text Solution
|
- If above tube is held vertical with open end upward then find the leng...
Text Solution
|
- An unknown gas behaves ideally at 540 K in low pressure region, then c...
Text Solution
|
- A flask has 10 molecules out of which four molecules are moving at 7 m...
Text Solution
|
- Two flask of equal volume are connected by a narrow tube (of negligibl...
Text Solution
|
- The van der Waal's constants for a gas are a=1.92 atm L^(2) "mol"^(-2)...
Text Solution
|
- Calculate the mole present of N(2) gas in a mixture of N(2) and O(2) i...
Text Solution
|
- Some gas is present in J-shaped tube and in this case level of mecury ...
Text Solution
|
- The density of a mixturee of O(2) and N(2) gases at 1 atm and 273 K is...
Text Solution
|
- One litre gaseous mixture is effused in 4.5 minutes and 30 seconds whi...
Text Solution
|
- Gas A taken in a closed rigid container is allowed to decompose partia...
Text Solution
|
- For a real gas critical pressure is 75 atm and van der Waal's constant...
Text Solution
|
- 2 moles of NO(g) and 16 gm of O(2)(g) were mixed in a 6.25 litre vesse...
Text Solution
|
- If the mean free path is 10 cm at one bar pressure, then, its value in...
Text Solution
|
- Average translational kinetic energy of an ideal gas molecule at 27^(@...
Text Solution
|
- H(2) and O(2) are kept in mass ratio 1:8 respectively at 6 atm. If sma...
Text Solution
|
- Calulate the mole fraction of N(2) gas in a mixture of N(2) and O(2). ...
Text Solution
|
- If at 200 K and 500 atm, density of CH(4) is 0.246 g//mL then its comp...
Text Solution
|
- Density of ideal gas at 2.46 atm and 300 K is 0.8 g//L Hence g-molar m...
Text Solution
|