Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GASEOUS STATE-Exercise
- If in below diagram after opening valve, final pressure is(7)/(6) Mpa,...
Text Solution
|
- An ideal gas occupies 2 litres volume at 300 K and 1 atm. Calculate th...
Text Solution
|
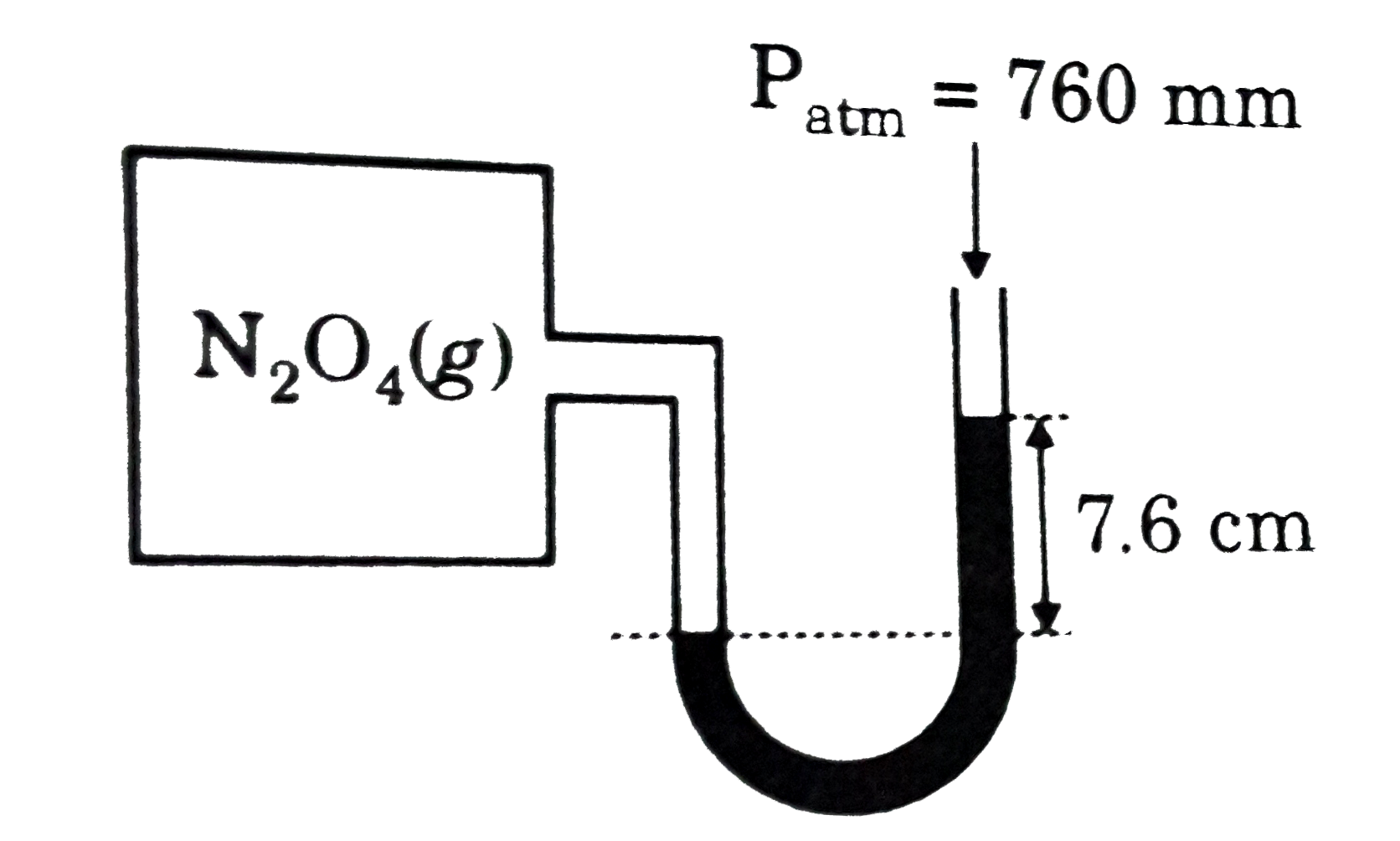
- In a container initially only N(2)O(4) is present and no difference in...
Text Solution
|
- At 30^(@)C dry air [75 % N(2)+25 % O(2)] is placed over H(2)O(l) at 80...
Text Solution
|
- Inifinite number of flasks are connected to one another as shown above...
Text Solution
|
- In 1 litre rigid vessel at 1 atm and 300 K, N "collision/sec-cm"^(2) o...
Text Solution
|
- 0.5 L of evacuated container is filled by gas upto 1 atm exactly, by c...
Text Solution
|
- At constant pressure mean free path of ideal gas lamda prop T^(x). Hen...
Text Solution
|
- 100 mL of a gas is stored over mercury in mercury manometer at 27^(@)C...
Text Solution
|
- A good vacuum produced in common lab apparatus corresponds to 10^(-6) ...
Text Solution
|
- An ideal gas at 650 Torr occupies a bulb of unknown volune. A certain ...
Text Solution
|
- On litre flask contains air, water vapour and a small amount of liquid...
Text Solution
|
- A diver at a depth of 10 m exhales a bubble of air of volume 24.63 mL....
Text Solution
|
- The gas 'A' decomposes as A rarr B + 5C A partially decomposed gaseo...
Text Solution
|
- A bulb of constant volume is attached to a very thin manometer tube as...
Text Solution
|
- For oxygen at 25^(@)C, the collision diameter is 0.361 nm. What is the...
Text Solution
|
- Two flasks A and B of equal volume containing NH(3) and HCl gases, are...
Text Solution
|
- 22.4 L CH(4) at 1 atm and 273 K was thought to have mass 16 g but when...
Text Solution
|
- Two glass bulbs A and B are connected by a very small tube having a st...
Text Solution
|
- A mixture of H(2),He and O(2) with mass ratio equal to the ratio of th...
Text Solution
|