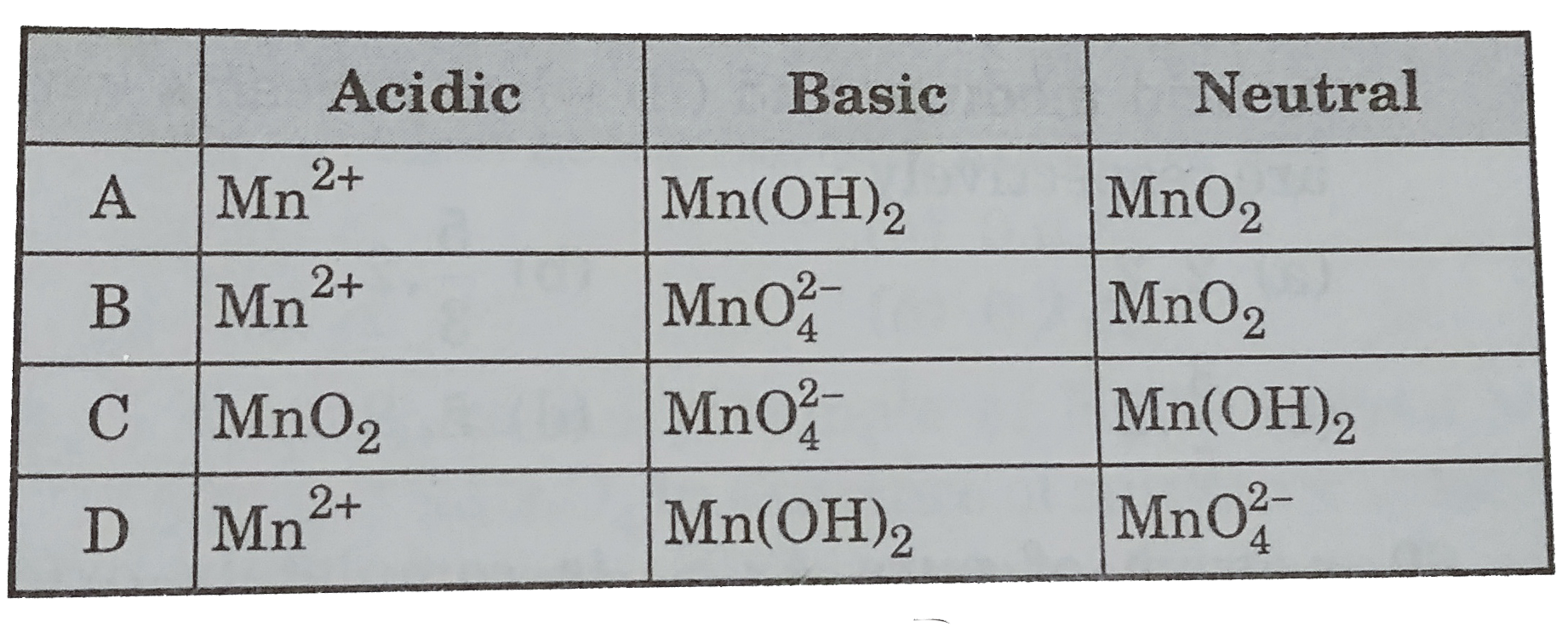
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-REDOX REACTIONS-All Questions
- 0.7g of (NH(4))(2)SO(4) sample was boiled with 100mL of 0.2 N NaOH sol...
Text Solution
|
- A mixutre solution of KOH and Na2CO3 requires 15 " mL of " (N)/(20) HC...
Text Solution
|
- When the permanganate ion, MnO(4)^(-),acts as an oxidizing agent it fo...
Text Solution
|
- 10 mL of 1 NHCl is mixed with 20 mL of 1MH(2)SO(4) and 30 mL of 1M NaO...
Text Solution
|
- H(2)O(2) acts as both oxidising and reducing agent. As oxidising agent...
Text Solution
|
- 1.2g of carbon is burnt completely in oxygen (limted supply) to produc...
Text Solution
|
- The valency factor of I(2) when, (i) it is formed by the reaction of p...
Text Solution
|
- x gram of pure As(2)S(3) is completely oxididsed to respective highest...
Text Solution
|
- The number of moles of oxalate ions oxidised by one mole of MnO(4)^(-)...
Text Solution
|
- What volume of 0.1M H(2)O(2) solution will be required to completely r...
Text Solution
|
- Calculate the number of millimoles of SO(2) if in the reaction,10mL of...
Text Solution
|
- 10 moles of ferric oxalate is oxidised by x mole of MnO(4)^(-) in acid...
Text Solution
|
- In iodometric estimation of Cu^(2+) ion, the following reaction took p...
Text Solution
|
- Calculate the moles of KMnO(4) required to react completely with 2 m a...
Text Solution
|
- 4:3 gm of an alkane is burnt in sufficient oxygen. The CO2 formed reac...
Text Solution
|
- What is the correct relation between normality (N) and molarity (M) of...
Text Solution
|
- Number of moles of electorns in the reaction during formation of 1 mol...
Text Solution
|
- 25 ml of (N)/(10) caustic soda solution exactly neuralised 20ml of an ...
Text Solution
|
- 10mL of H(2)O solution weighs 10 gm. The solution is diluted to 250mL....
Text Solution
|
- 0.8 M FeSO(4) solution requires 160mL,0.2 M Al(2)(Cr(2)O(7))(3) in aci...
Text Solution
|