Similar Questions
Explore conceptually related problems
Recommended Questions
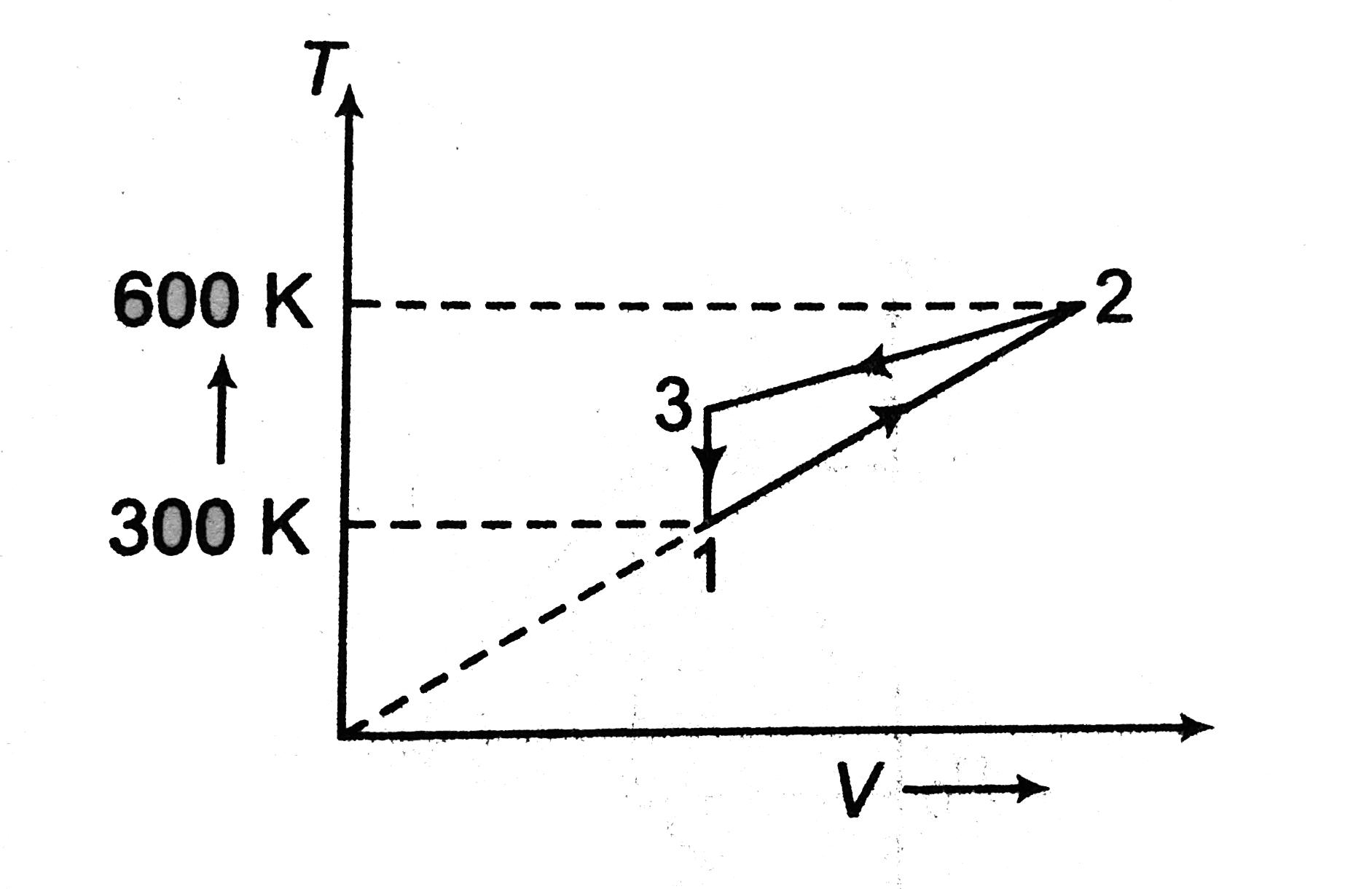
- Two moles of an ideal gas are undergone a cyclic process 1-2-3-1. If n...
Text Solution
|
- Two moles of an ideal gas are undergone a cyclic process 1-2-3-1. If n...
Text Solution
|
- On a T-P diagram, two moles of ideal gas perform process AB and CD. If...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process, as sho...
Text Solution
|
- An ideal gas is taken in a cyclic process as shown in the figure. Calc...
Text Solution
|
- Two moles of helium gas undergo a cyclic process as shown in figure. A...
Text Solution
|
- एक मोल आदर्श गैस का रुद्वोष्म प्रक्रिया द्वारा ताप T(1) से T(2) तक बढ़...
Text Solution
|