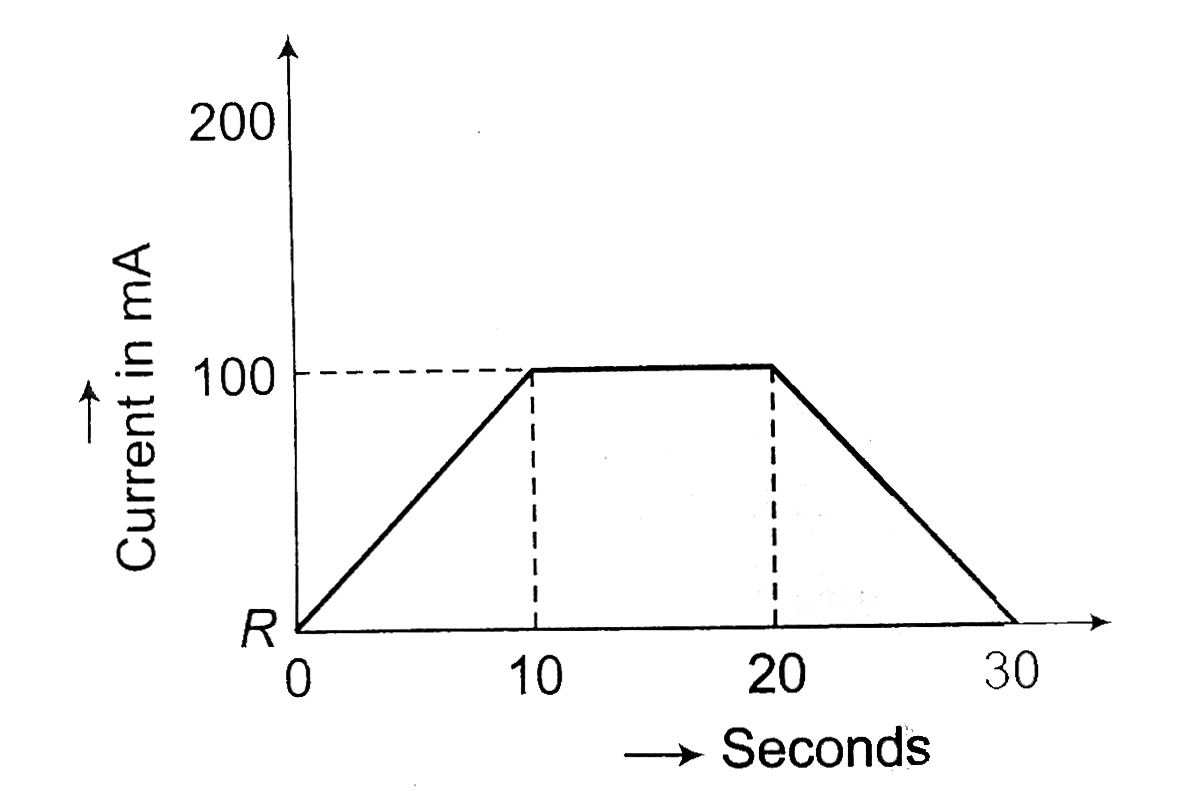
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEATING AND CHEMICAL EFFECT OF CURRENT
ERRORLESS |Exercise Heating Effect of Current|2 VideosHEATING AND CHEMICAL EFFECT OF CURRENT
ERRORLESS |Exercise Chemical Effect Current|1 VideosELECTROSTATICS
ERRORLESS |Exercise Ordinary Thinking Objective Questions (Electric Flux and Gauss.s Law)|28 VideosKINETIC THEORY OF GASES
ERRORLESS |Exercise QUESTIONS|126 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS -HEATING AND CHEMICAL EFFECT OF CURRENT-Self Evaluation Test
- In a copper voltmeter, mass deposite in 30 seconds is 'm' gram. If the...
Text Solution
|
- An electric kettle has two coils. When one of these is switched on, th...
Text Solution
|
- A 3^(0)C rise in temperature is observed in a conductor by passing a c...
Text Solution
|
- Two identical electric lamps marked 500 W, 220 V are connected in seri...
Text Solution
|
- When 1 gm hydrogen (e.c.e.=1.044xx10^(-8)kg//C) forms water, 34 kcal h...
Text Solution
|
- In how much time, one litre of H(2) will be collected by 5 A current ?...
Text Solution
|
- Figure 7.37 shows a network of three resistances. When some potential...
Text Solution
|
- If the length of the filament of a heater is reduced by 10%, the power...
Text Solution
|
- Find the thermo-emf developed in a copper-silver thermocopule when the...
Text Solution
|
- How much current should be passed through acidified water for 100 s to...
Text Solution
|
- The resistance of the filament of a lamp increases with the increase i...
Text Solution
|
- In the following circuit, 18 Omega resistor develops sec J/2 due to cu...
Text Solution
|
- If resistance of the filament increases with temperature, what will be...
Text Solution
|
- In an electroplating experiment, m gm of silver is deposited when 4 am...
Text Solution
|
- Two bulbs consume same energy when operated at 200 V and 300 V , respe...
Text Solution
|