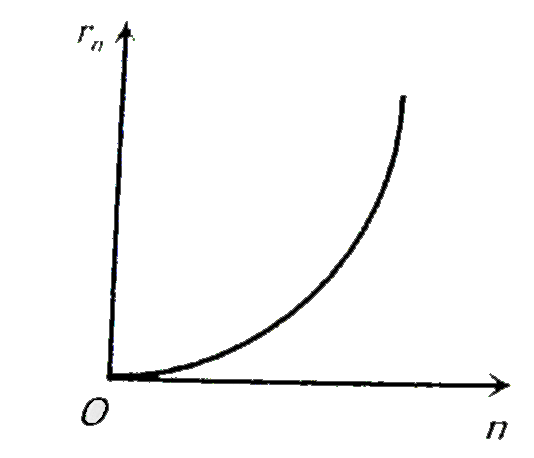
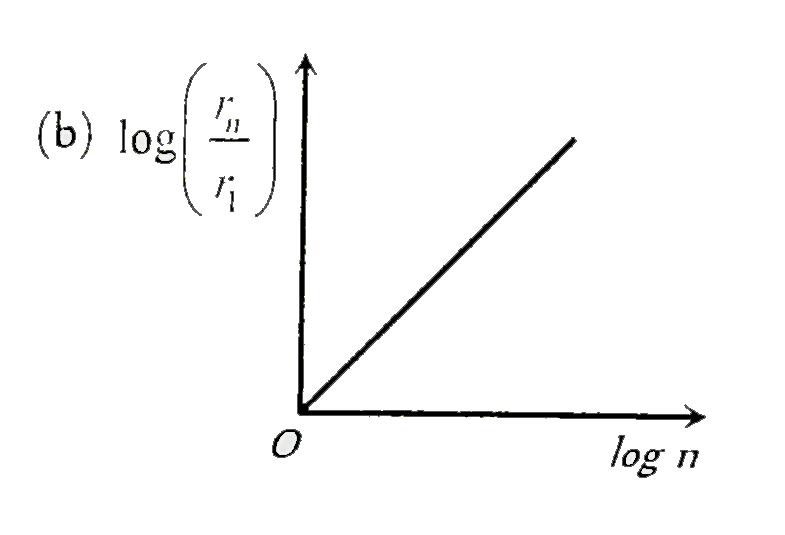
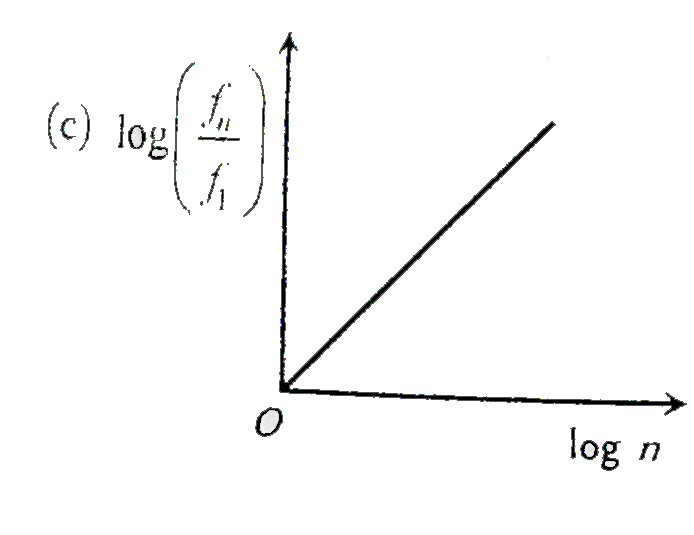A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC AND NUCLEAR PHYSICS
ERRORLESS |Exercise Assertion and Reason|1 VideosATOMIC AND NUCLEAR PHYSICS
ERRORLESS |Exercise S ET|1 VideosATOMIC AND NUCLEAR PHYSICS
ERRORLESS |Exercise Nuckeus, Nuclear Reaction|2 VideosALTERNATING CURRENT
ERRORLESS |Exercise SET|15 VideosCOMMUNICATION SYSTEM
ERRORLESS |Exercise S E T|14 Videos
Similar Questions
Explore conceptually related problems