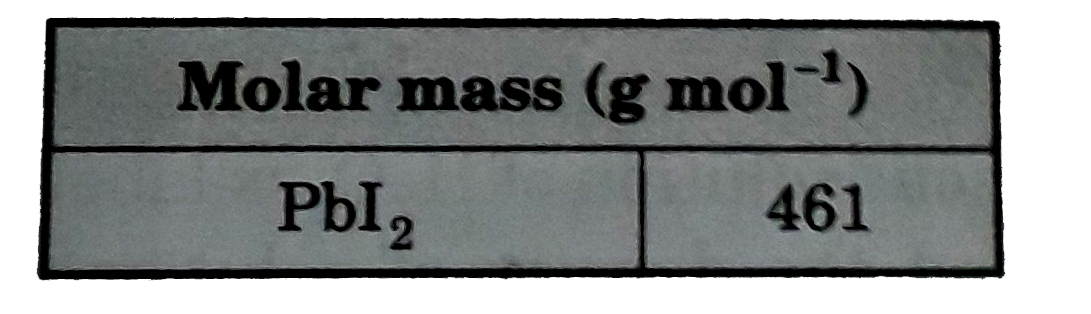A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS
GRB PUBLICATION|Exercise Percentage labelling of Oleum sample, volume strength of hyrogen Peroxide, ppm|23 VideosMOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS
GRB PUBLICATION|Exercise K. Eudiometry|74 VideosMOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS
GRB PUBLICATION|Exercise Experimental Methods|24 VideosISOMERISM
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|67 VideosNOMENCLATURE AND CLASSIFICATION
GRB PUBLICATION|Exercise Subjective Type|24 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS-Concentration Terms
- 40.0g of a solute is dissolved in 500mL of solvent to give a solution ...
Text Solution
|
- A 49.9g sample of barium hydroxide octahydrate, Ba(OH)(2).8H(2)O is di...
Text Solution
|
- What is the maximum mass of PbI(2) that can be precipitated by mixing ...
Text Solution
|
- Commercial vinegar is a 5.00% by mass aqueous solution of acetic acid,...
Text Solution
|
- What is the molarity of Na^(+) ions in a solution made by dissolving 4...
Text Solution
|
- What is the molarity of a hydorchlric acid solution if 20.00 mL of it ...
Text Solution
|
- A 65.25 g sample fo CuSO(4).5H(2)O (M=249.7) is dissolved in enough wa...
Text Solution
|
- How many moles of sulphate ions are in 100 mL of a solution of 0.0020 ...
Text Solution
|
- What is the molality of a solution made by dissolving 36.0g of glucose...
Text Solution
|
- What is the final [Na^(+)] in a solution prepared by mixing 70.0mL of ...
Text Solution
|
- The active ingredient in commercial bleach is sodium hypochloride, NaO...
Text Solution
|
- What mass of NaHCO(3) (M=84.0) is required to completely neutralize 25...
Text Solution
|
- Which mixture of water and H(2)SO(4) represents a soltion with a conce...
Text Solution
|
- What is the mole fraction of CH(3)OH in an aqueous solution that is 12...
Text Solution
|
- A solution is prepared by mixing 25.0 mL of 6.0 M HCI with 45.0 mL of ...
Text Solution
|
- A 25.0 mL sample of 0.15 M silver nitrate, AgNO(3), is reacted with a ...
Text Solution
|
- What volume of 95% H(2) SO(4) by weight (d = 1.85 g mL^(-1)) and what ...
Text Solution
|
- Equal weight of NaCl and KCl are dissolved separately in equal of solu...
Text Solution
|
- The strength of H(2)SO(4) solution (in gm/litre), 12 ml of which neutr...
Text Solution
|
- 120 gm of glucose is dissolved to make 1 litre solution having density...
Text Solution
|