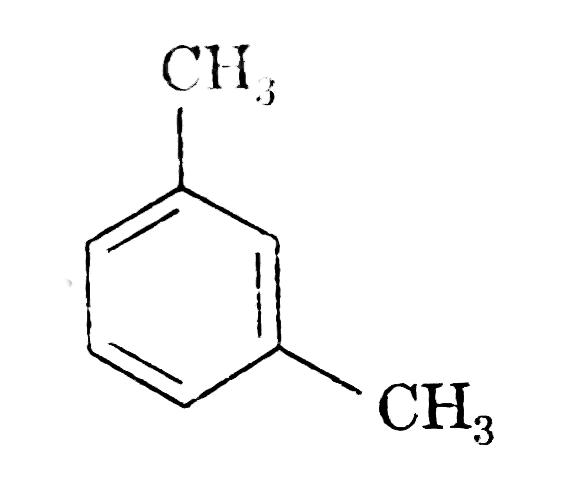
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS
GRB PUBLICATION|Exercise Reasoning type|24 VideosMOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS
GRB PUBLICATION|Exercise Multiple objective type|23 VideosMOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS
GRB PUBLICATION|Exercise Percentage labelling of Oleum sample, volume strength of hyrogen Peroxide, ppm|23 VideosISOMERISM
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|67 VideosNOMENCLATURE AND CLASSIFICATION
GRB PUBLICATION|Exercise Subjective Type|24 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS-K. Eudiometry
- How many litres of oxygen at 1 atm and 273 K will be required to burn ...
Text Solution
|
- When a certain amount of octane, C8H18 is burnt completely 7.04 gm CO2...
Text Solution
|
- An ideal gaseous mixture of ethane (C(2)H(6)) and ethene (C(2)H(4)) oc...
Text Solution
|
- combustion gives CO2(g) and H2O(g).Find ratio of number of atoms O to ...
Text Solution
|
- C(6)H(5)OH(g)+O(2)rarrCO(2)(g) +H(2)O(l) Magnitude of volume change ...
Text Solution
|
- Methyl-t-butel ether, C5H12O is added to gasoline to promote cleaner b...
Text Solution
|
- The reaction of ethanol, C2H5OH , with oxygen is a popular classroom d...
Text Solution
|
- The mass of 560 cm^3 of a gas at 0^@C and 1 atm is 1.60 g. Which gas c...
Text Solution
|
- Assume 0.10 L of N2 and 0.18 L of H2, both at 50 atm and 450^@C, are r...
Text Solution
|
- Ethanol burns in excess oxygen to form CO2(g) and H2O(g) according to ...
Text Solution
|
- Acetylene, C2H2 reacts with oxygen according to the unbalanced equatio...
Text Solution
|
- Which combustion prouduct is produced THE LEAST by gasoline-powered ve...
Text Solution
|
- A 12 gm sample of CH4 and C(2)H(4) on complete oxidation with O2 for...
Text Solution
|
- Toluence, C7H8 is added to gasoline to increase its octane rating. Wha...
Text Solution
|
- Which absorbs gaseous carbon dioxide most effectively?
Text Solution
|
- Methylamine, CH3NH2, reacts with O2 (in moles) is required to react co...
Text Solution
|
- 100 L of carbon dioxide measured at 740 mm Hg and 50^(@)C is produced ...
Text Solution
|
- A 10.0 g sample of an oxide of copper forms metallic copper and 1.26 g...
Text Solution
|
- A 10.00g sample of a compound containing C,H,and O is burned completel...
Text Solution
|
- How many moles of water are produced by the complete comustion of 14.4...
Text Solution
|