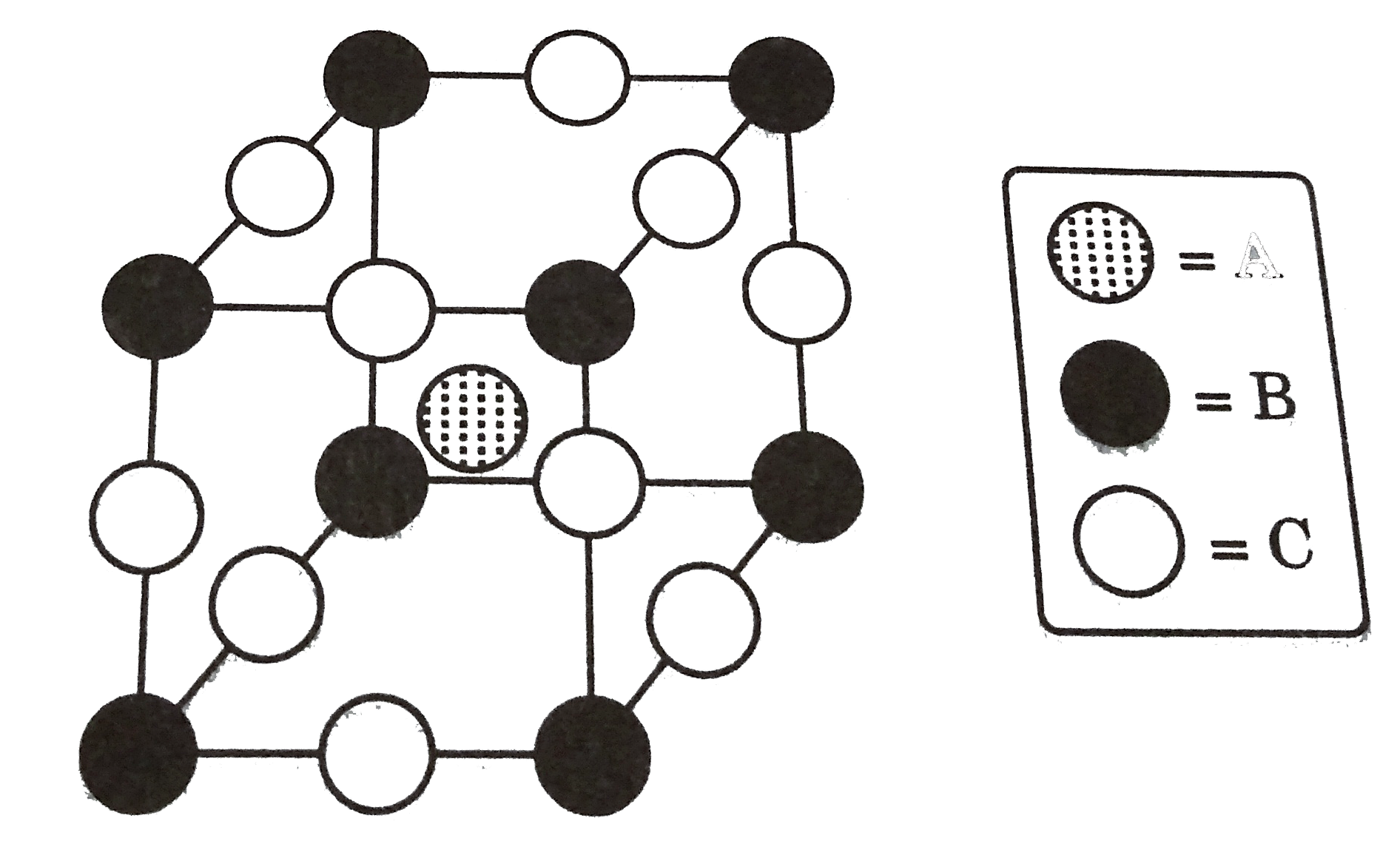
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SOLID STATE-All Questions
- The density of KBr is 2.75 gm//c c length of the unit cell is 654pm ( ...
Text Solution
|
- A solid element (monoatomic) exists as cubic crystal. If its atomic ra...
Text Solution
|
- The cubic unit cell of a perovakite structure containing atoms of type...
Text Solution
|
- Three elements P,Q and R crystallize in a cubic solid lattice. The P a...
Text Solution
|
- Consider a cube 1 of body-centered cubic unit cell of edge length 'a'....
Text Solution
|
- In a face centerd lattice of X and YX atoms are present at the corners...
Text Solution
|
- A compound of A and B crystallizes in a cubic lattice in which A atoms...
Text Solution
|
- In a solid AB having the NaCl structure, A atom occupies the corners o...
Text Solution
|
- An ionic compound has a unit cell consisting of A ions at the corners ...
Text Solution
|
- A substance A(x)B(y) crystallizes in a face-centred cubic lattice in w...
Text Solution
|
- A crystal is made up of particals X, Y, and Z. X froms fcc packing. Y ...
Text Solution
|
- In a face-centered lattice of X and Y, X atoms are present at the corn...
Text Solution
|
- In a cubic structure of compound which is made from X and Y, where X a...
Text Solution
|
- In a face centered lattice of X and Y, X atoms are present at the corn...
Text Solution
|
- In a C CP lattice of X and Y atoms are present at the corners while Y ...
Text Solution
|
- A solid has a structure in which W atoms are located at the corners of...
Text Solution
|
- Find the lowest possible empirical formular in an arrangements of unit...
Text Solution
|
- The volume of atom present in a face-centred cubic unit cell of a meta...
Text Solution
|
- Percentage of free space in cubic close packed structure and in body c...
Text Solution
|
- In a face centerd cubic cell, the contribution of an atom at a face of...
Text Solution
|