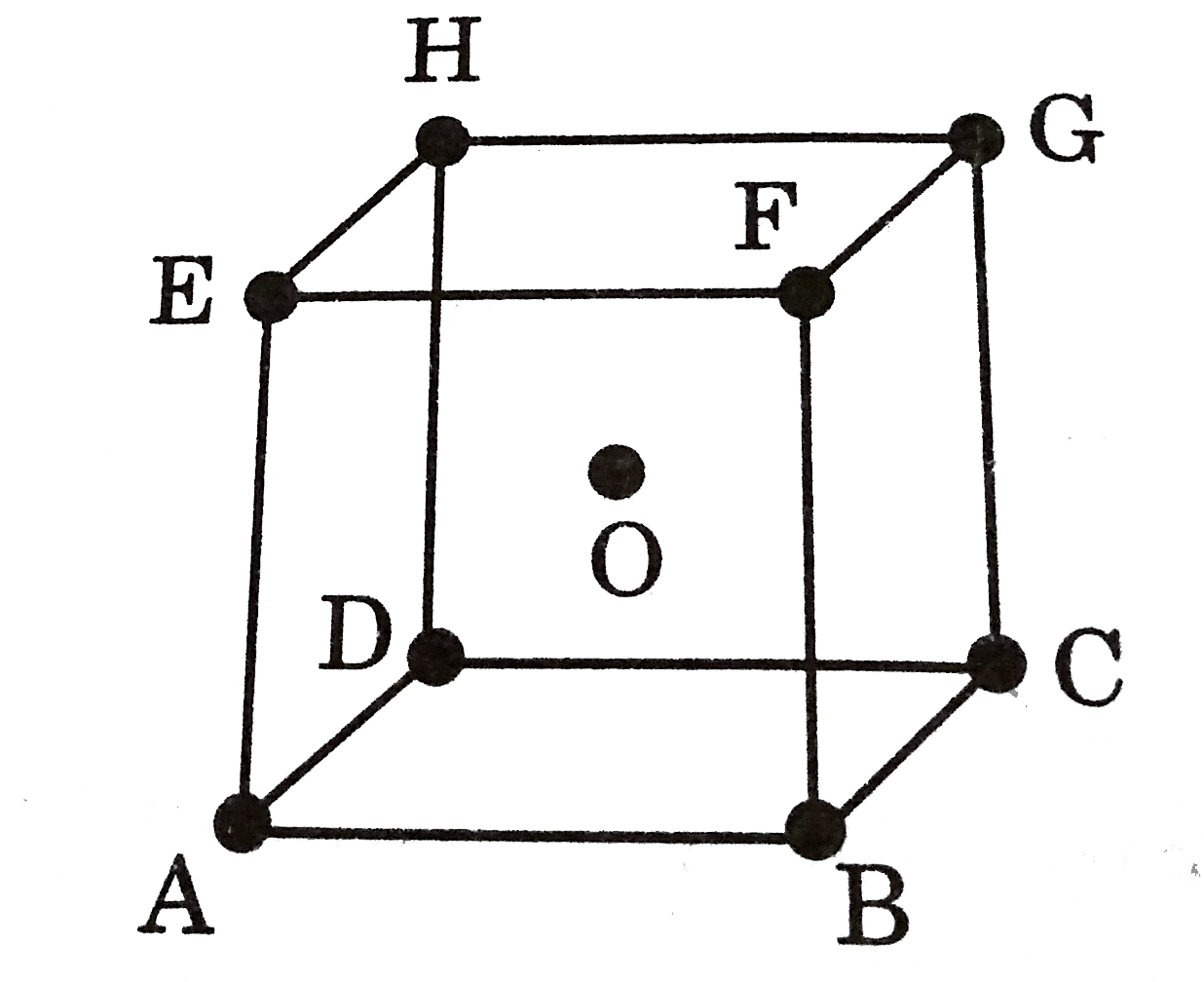
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SOLID STATE-All Questions
- In a face centerd cubic cell, the contribution of an atom at a face of...
Text Solution
|
- An fcc lattice has a lattice parameter a = 400 pm. Calculater the mola...
Text Solution
|
- A body centred cubic arrangement is shown below: O is the body ce...
Text Solution
|
- In a face centered cubic cell , an the face contributes in the unit ce...
Text Solution
|
- Copper crystallises in a structure of face centred cubic unit cell. Th...
Text Solution
|
- If the radius of a metal is 2.00Å and its crystal structure is in cubi...
Text Solution
|
- Platinum crystallize in a face-centred cubic crystal with a unite cell...
Text Solution
|
- A solid has a b.c.c. structure . If the distance of closest approach b...
Text Solution
|
- The compound AB crystallizes in cube lattice in which both the element...
Text Solution
|
- if a metal has a bcc crystal structure, the coordination number is 8,b...
Text Solution
|
- In a ccp structure, the :
Text Solution
|
- In a face centred cubic lattice the number of nearest neighbours for a...
Text Solution
|
- How manyu 'nearest' and 'next nearest' neighbours respectively does po...
Text Solution
|
- What is the maximum radius of the circle which can be kept in a two di...
Text Solution
|
- Which of the following options represents correctly matched value of n...
Text Solution
|
- Which of the following options represents correctly matched value of n...
Text Solution
|
- The closest distance between Si and C in SiC is 0.866Å. What will be t...
Text Solution
|
- In a BBC unit cell, fraction of face diagonal not covered by atoms is ...
Text Solution
|
- The only incorrect statement for the packing of identical spheres in t...
Text Solution
|
- In the body-centred cubic unit cell and face centred cubic unit cell, ...
Text Solution
|