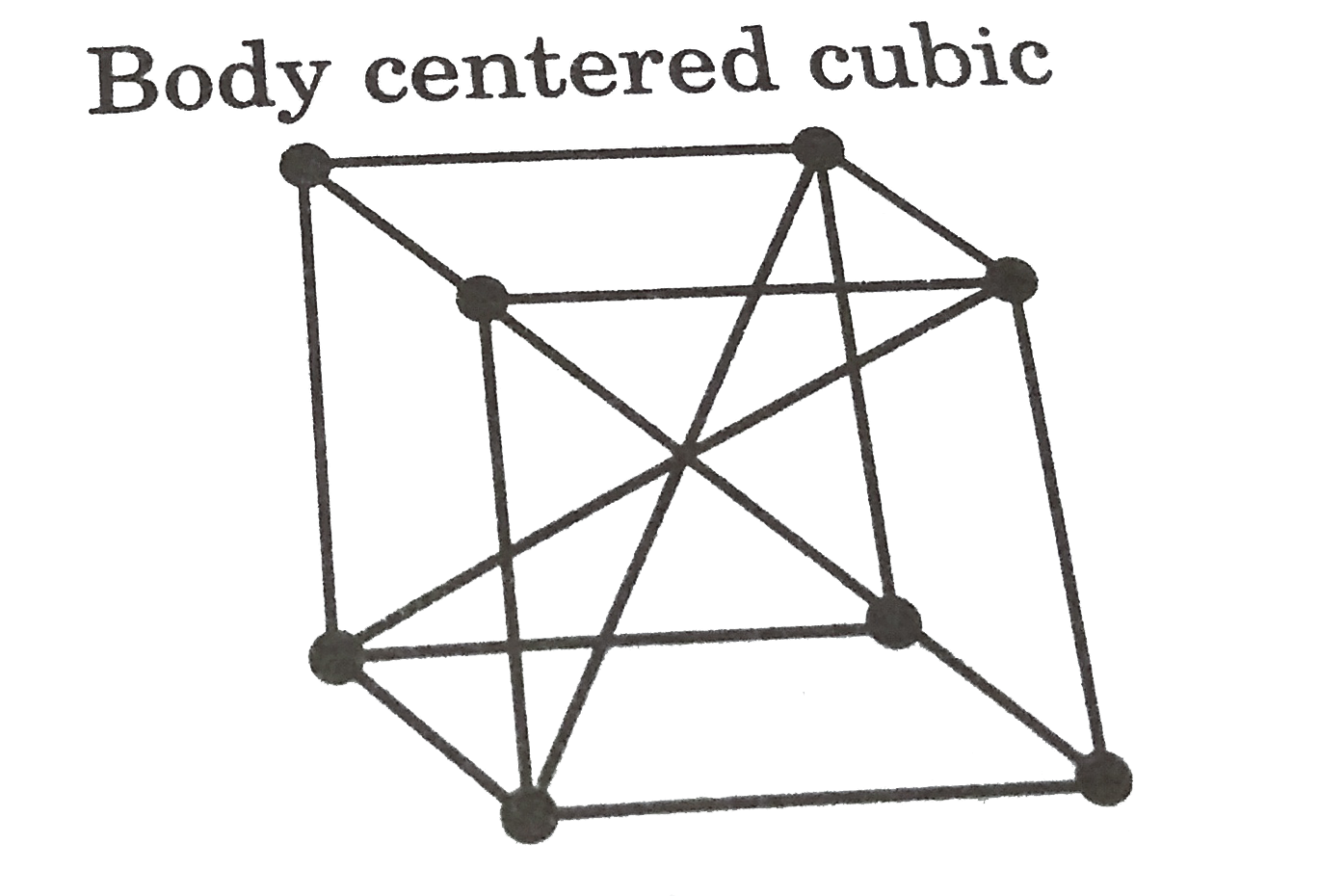
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SOLID STATE-All Questions
- C represents the height of the HCP unit cell and a represents the edge...
Text Solution
|
- Metallic gold crystallises in face centred cubic lattice with edge-len...
Text Solution
|
- Metallic sodium has a body-centred cubic unit cell. How many atoms are...
Text Solution
|
- The metal M crystallizes in a bocy centred lattice with cell edge 400p...
Text Solution
|
- How many nearest neighbours surrounded each particle in a face-centred...
Text Solution
|
- Consider the solids: body-centred cubic (bcc), face-centred cubic (fcc...
Text Solution
|
- Solid calcium occurs as either cubic closest packing or hexagonal clos...
Text Solution
|
- The atoms in crystals of silver metal are arranged in a cubic closest ...
Text Solution
|
- In a crystal of a typical metallic element, an atom has how many neare...
Text Solution
|
- What is the coordination number of each atom in a hexagonal close-pack...
Text Solution
|
- Which statement about atoms arranged in a body-centered cubic (bcc) cr...
Text Solution
|
- Packing fraction in F.C.C lattice is:
Text Solution
|
- If X = no. of second nearest neighbours of a metal atom which crystall...
Text Solution
|
- Of the three types of cubic lattices, which have the highest and lowes...
Text Solution
|
- A metal crystallizes in a body centred cubic lattice (bcc) with the ed...
Text Solution
|
- A solid crystallinses as cubic close packing of O^(2-) ions. A^(x+) io...
Text Solution
|
- Select the correct statements about FCC (ABCAB…) Structures.
Text Solution
|
- What will be approximate packing efficiency of the crystal which forms...
Text Solution
|
- Which of the following option(s) is//are incorrect if an arrangement i...
Text Solution
|
- Two elements A and B have atomic masses 40 and 60 respectively. If B f...
Text Solution
|