A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SOLID STATE-All Questions
- The number of octahedral and terhedral sides in a cubical closed packe...
Text Solution
|
- A mineral having the formula AB(2) crystallizes in the ccp lattice, wi...
Text Solution
|
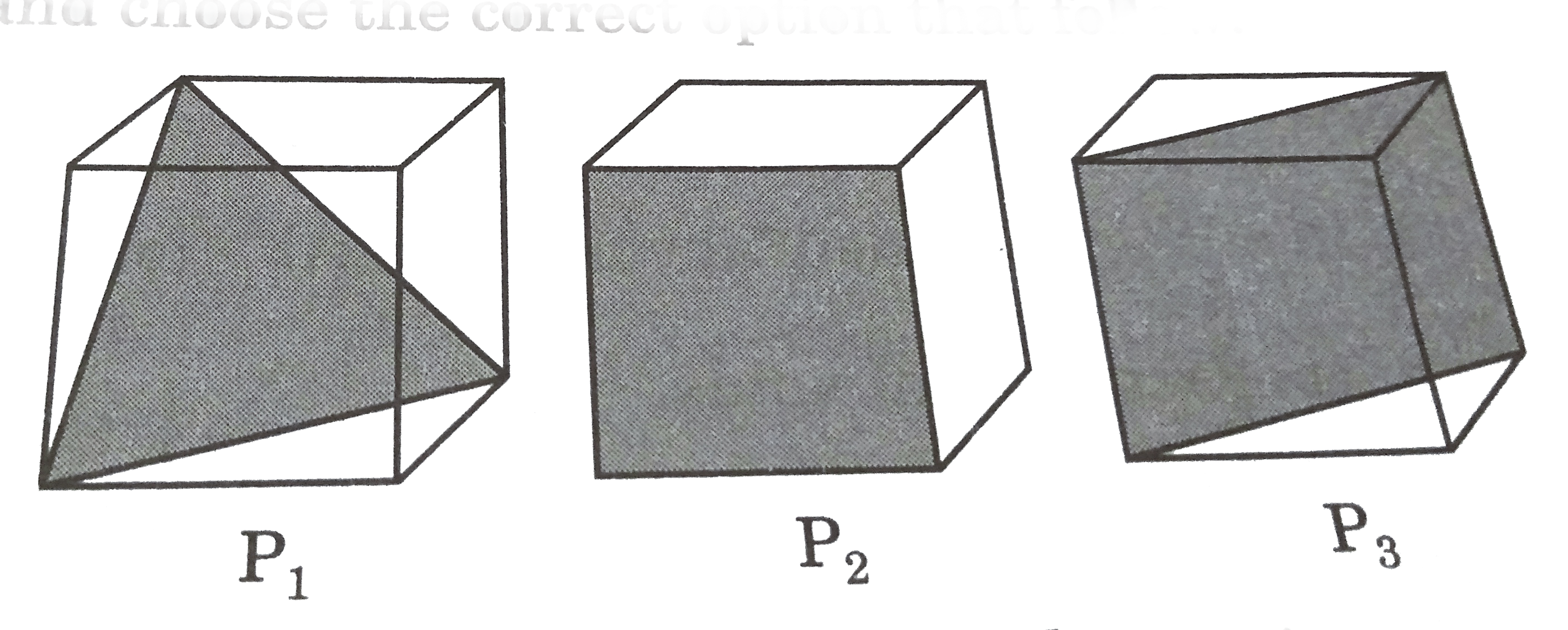
- Following three planes (P(1), P(2), P(3)) in an FCC unit cell are show...
Text Solution
|
- Given an alloy of Cu, Ag and in which Cu atoms consists the CCP arrang...
Text Solution
|
- What is the packing fraction close packed cylinders?
Text Solution
|
- In the closest packing of atoms
Text Solution
|
- Correct statement for ccp is:
Text Solution
|
- The empty space between the shaded balls and hallow balls as shown in ...
Text Solution
|
- In a FCC unit cell x = distance between two nearest O.V. y = dista...
Text Solution
|
- Length of body diagonal in FCC unit cell is alpha Å. Distance between ...
Text Solution
|
- The shortest distance between an octahedral and tetrahedral void in F....
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown b...
Text Solution
|
- Let the height of hcp unit cell is 'h'. The height of octahedral voids...
Text Solution
|
- In NaCl if r(Na+) = 100pm, then maximum size of r(Cl-) will be:
Text Solution
|
- How many unit cells are present in a cube-shaped crystal of NaCl of ma...
Text Solution
|
- The unit cell cube length for LiCl(just like NaCl structures) is 5.14Å...
Text Solution
|
- The numberof next nearest neighbours of Cs^(+) in a lattice of CsCl is...
Text Solution
|
- 4.2 gm of carbonate of an alkaline earth metal is dissolved in excess ...
Text Solution
|
- CsBr has bcc like structures with edge length 4.3Å. The shortest inter...
Text Solution
|
- BaO has a rock-salt type structure. When subjected to high pressure, t...
Text Solution
|