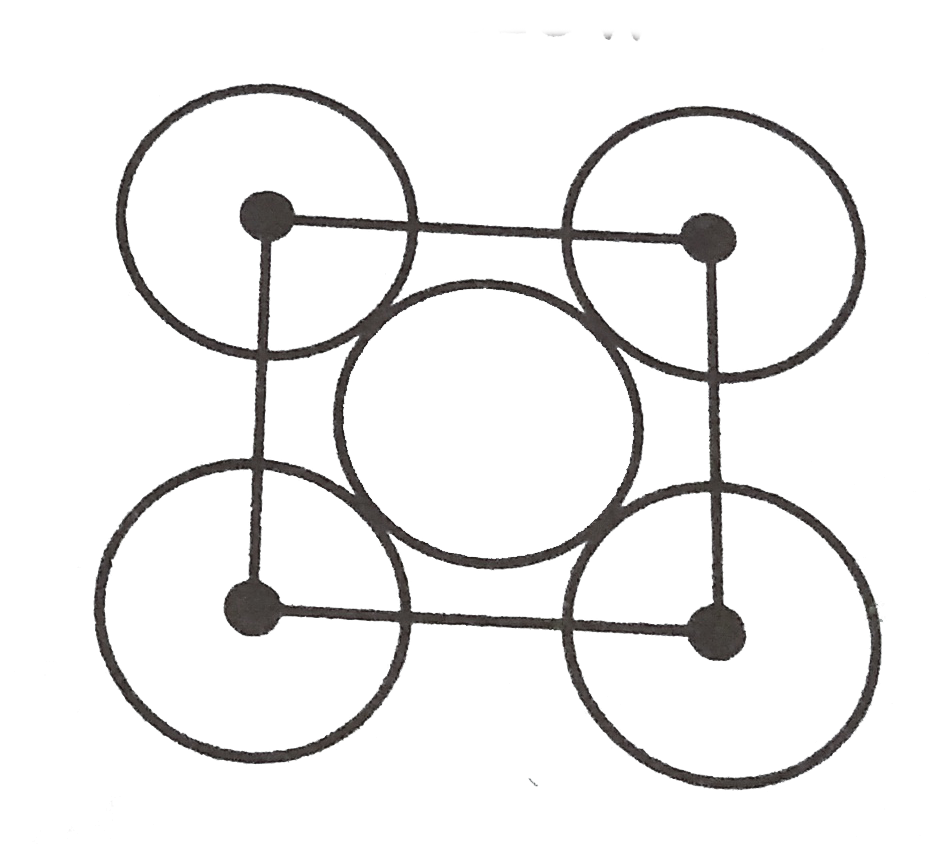
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SOLID STATE-All Questions
- Length of body diagonal in FCC unit cell is alpha Å. Distance between ...
Text Solution
|
- The shortest distance between an octahedral and tetrahedral void in F....
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown b...
Text Solution
|
- Let the height of hcp unit cell is 'h'. The height of octahedral voids...
Text Solution
|
- In NaCl if r(Na+) = 100pm, then maximum size of r(Cl-) will be:
Text Solution
|
- How many unit cells are present in a cube-shaped crystal of NaCl of ma...
Text Solution
|
- The unit cell cube length for LiCl(just like NaCl structures) is 5.14Å...
Text Solution
|
- The numberof next nearest neighbours of Cs^(+) in a lattice of CsCl is...
Text Solution
|
- 4.2 gm of carbonate of an alkaline earth metal is dissolved in excess ...
Text Solution
|
- CsBr has bcc like structures with edge length 4.3Å. The shortest inter...
Text Solution
|
- BaO has a rock-salt type structure. When subjected to high pressure, t...
Text Solution
|
- For an ionic solid of the general formula AB and coordination number 6...
Text Solution
|
- The radius of Ag^(+) ion is 126 pm and that of I^(-)ion is 216 pm. The...
Text Solution
|
- The tetrahedral voids formed by ccp arrangement of Cl^(-) ions in rock...
Text Solution
|
- Which of the expressions is correct in the case of a sodium chloride u...
Text Solution
|
- MgO exists in a rock- salt type unit cell .Each Mg^(+2) ion will be is...
Text Solution
|
- How many units cells are there in 1.00g cube shpaed ideal crystal of A...
Text Solution
|
- The ionic radii of Rb^(+) and I^(-) are 1.46 and 2.16Å. The most proba...
Text Solution
|
- The edge length of a face contact with each other ionic substance is 5...
Text Solution
|
- Which of the following statements is correct in the rock-salt structur...
Text Solution
|