A
B
C
D
Text Solution
Verified by Experts
GRB PUBLICATION-SOLID STATE-All Questions
- In a ideal crystal there nust be regular repeating arrangement of the ...
Text Solution
|
- In a ideal crystal there nust be regular repeating arrangement of the ...
Text Solution
|
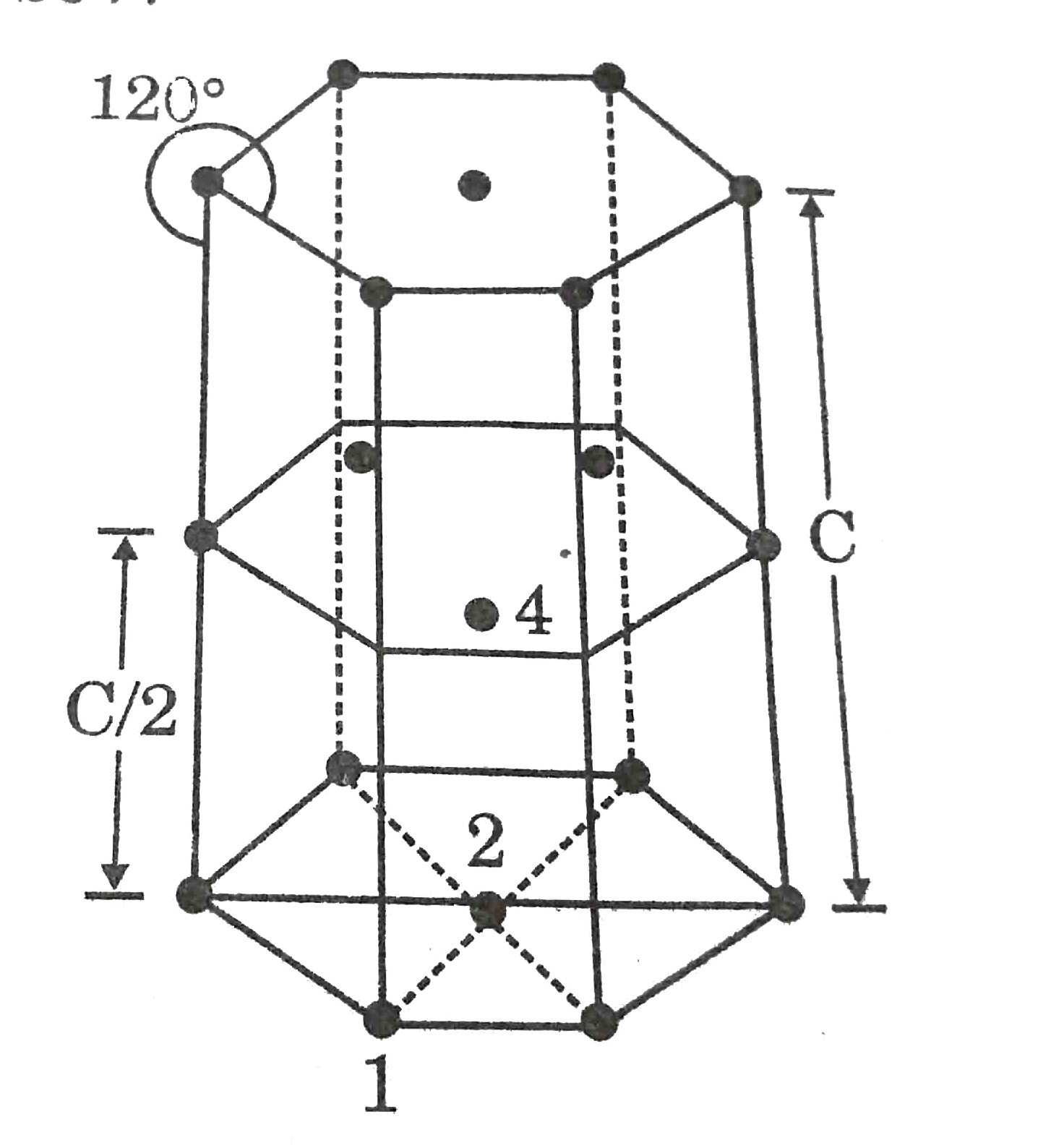
- In hexagonal system of crystals, a frequently encountered arrangement ...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- In a hexaonal system system of cycstals, a frequently encountered arra...
Text Solution
|
- Match list-I with list-II and select the correct answer by using the c...
Text Solution
|
- Match the following columns:
Text Solution
|
- Match the crystal system/unit, cells mentioned in Column-I with their ...
Text Solution
|
- The density of solid argon is 1.65g//mL at -233^(@)C . If the argon a...
Text Solution
|
- Calculate packing fraction of CsCI structure. Use : sqrt(3) = 1.732, p...
Text Solution
|
- Calculate radius of an atom (in Å) the crystal which has a density equ...
Text Solution
|
- An ionic compound (A^(+)B^(-)) crystallizes in rock salt structure. If...
Text Solution
|
- The difference in coordination numbers of hexagonal close packing in 3...
Text Solution
|
- Calculate the edge length of the unit cell of sodium chloride given de...
Text Solution
|
- The density of solid argon is (2)/(3) ("amu"//Å^(3)) at 40K. If the Ar...
Text Solution
|
- A mineral of iron contains an oxide containing 72.36% iron by mass and...
Text Solution
|
- An ionic solid AB(2) isomorphous to the rutile structure (a tetragonal...
Text Solution
|
- In an ionic solid r((+))=1.6A and r((-))=1.864A. Use the radius ratio...
Text Solution
|
- There are 5.6 xx 10^(24) unit cells in 1 kg of metal for which the den...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At the low temperature at whi...
Text Solution
|