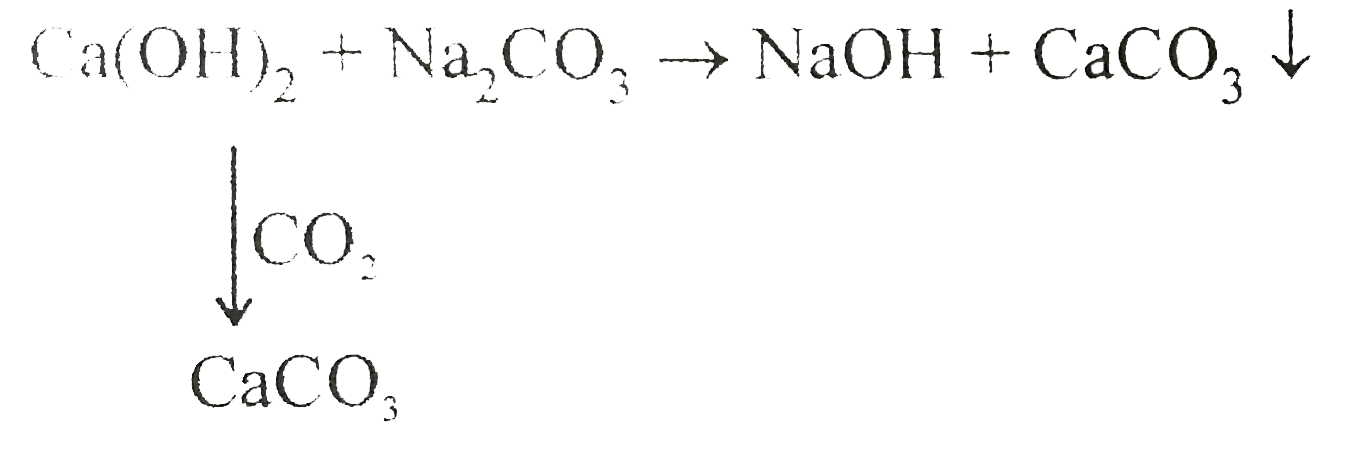A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-HYDROGEN-All Questions
- H(2)O(2) does not act as
Text Solution
|
- The structure of H(2)O(2) is
Text Solution
|
- Chemical a is used for water softening to remove temporary hardness. A...
Text Solution
|
- Permanent hardness due to Mg^(2+) ions is best removed by
Text Solution
|
- Permutit is :
Text Solution
|
- Determine the degree of hardness of a sample of water containing 30 pp...
Text Solution
|
- Deutero methane is obtained by the deuterolysis of
Text Solution
|
- When a substance A reacts with water it produces a combustible gas B a...
Text Solution
|
- What is false about H(2)O(2) ?
Text Solution
|
- In which of the following reactions does hydrogen act as an oxidising ...
Text Solution
|
- Mass precentage of deuterium in heavy water is
Text Solution
|
- 2g of aluminium is treated, separately with excess of dilute H(2)SO(4)...
Text Solution
|
- In which of the solution hydrogen peroxide neither acts as oxidising a...
Text Solution
|
- Which one of the following reactions does not form gaseous product ?
Text Solution
|
- In alkaline medium, H(2)O(2) reacts with Fe^(3+) and Mn^(2+) separatel...
Text Solution
|
- Hardness producing salt, whose solubility in water decreases with rise...
Text Solution
|
- Hydrolysis of one mole of peroxy disulphuric acid produces
Text Solution
|
- A sample of water containing some dissolved table sugar and common sal...
Text Solution
|
- Water obtained by purification with organic ion exchange resins is
Text Solution
|
- When two ice cubes are pressed over each other, they unite to form one...
Text Solution
|