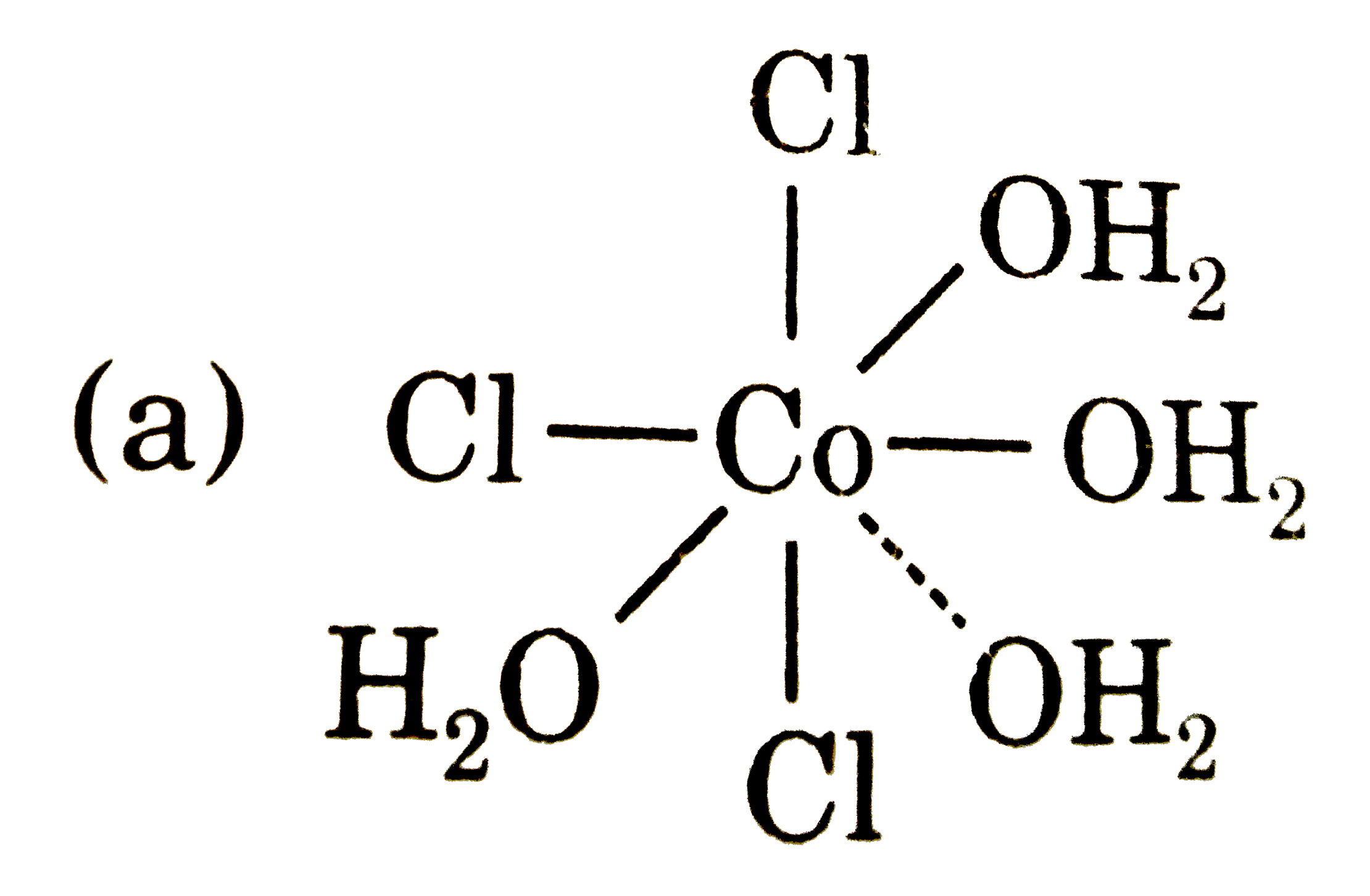
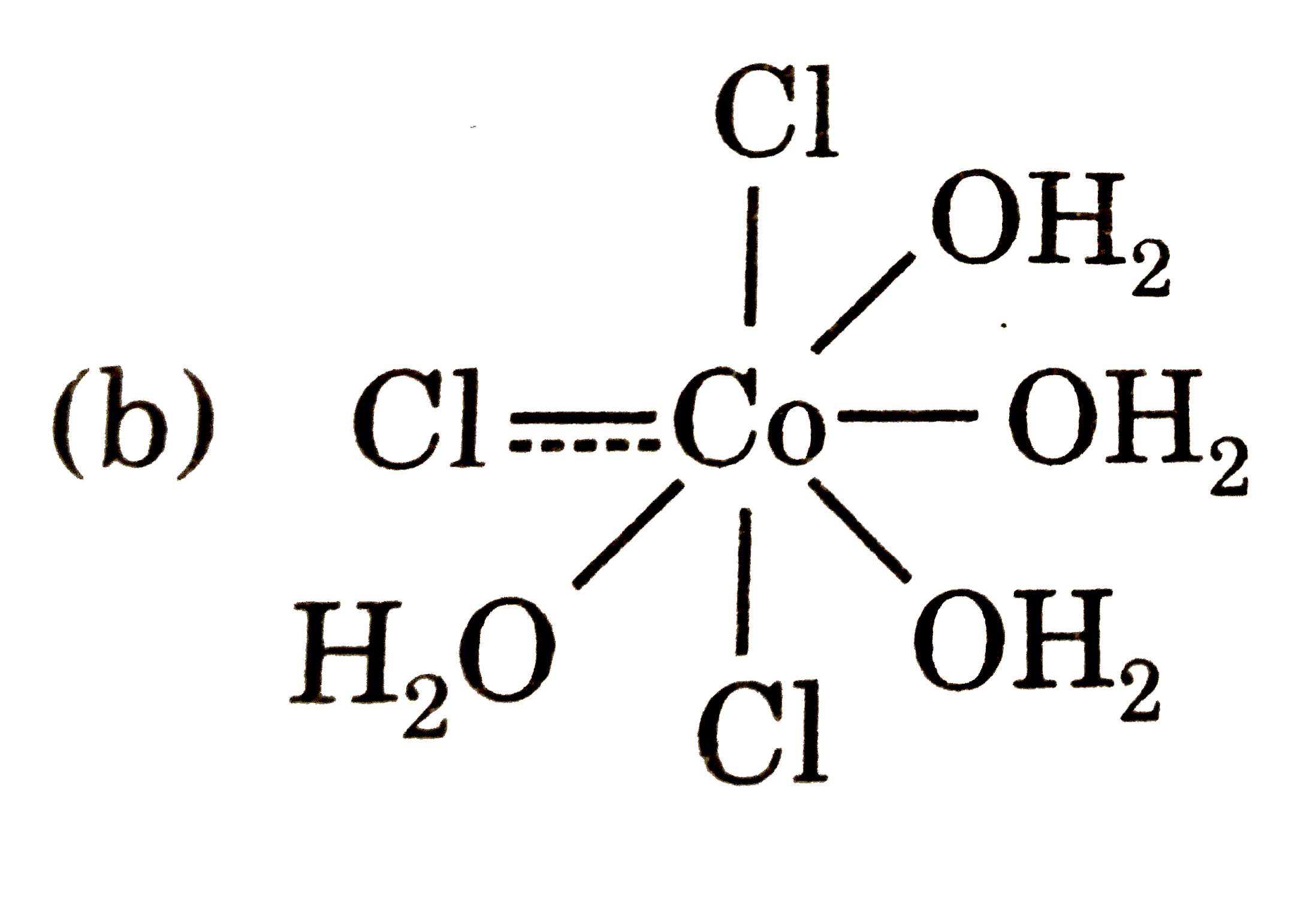
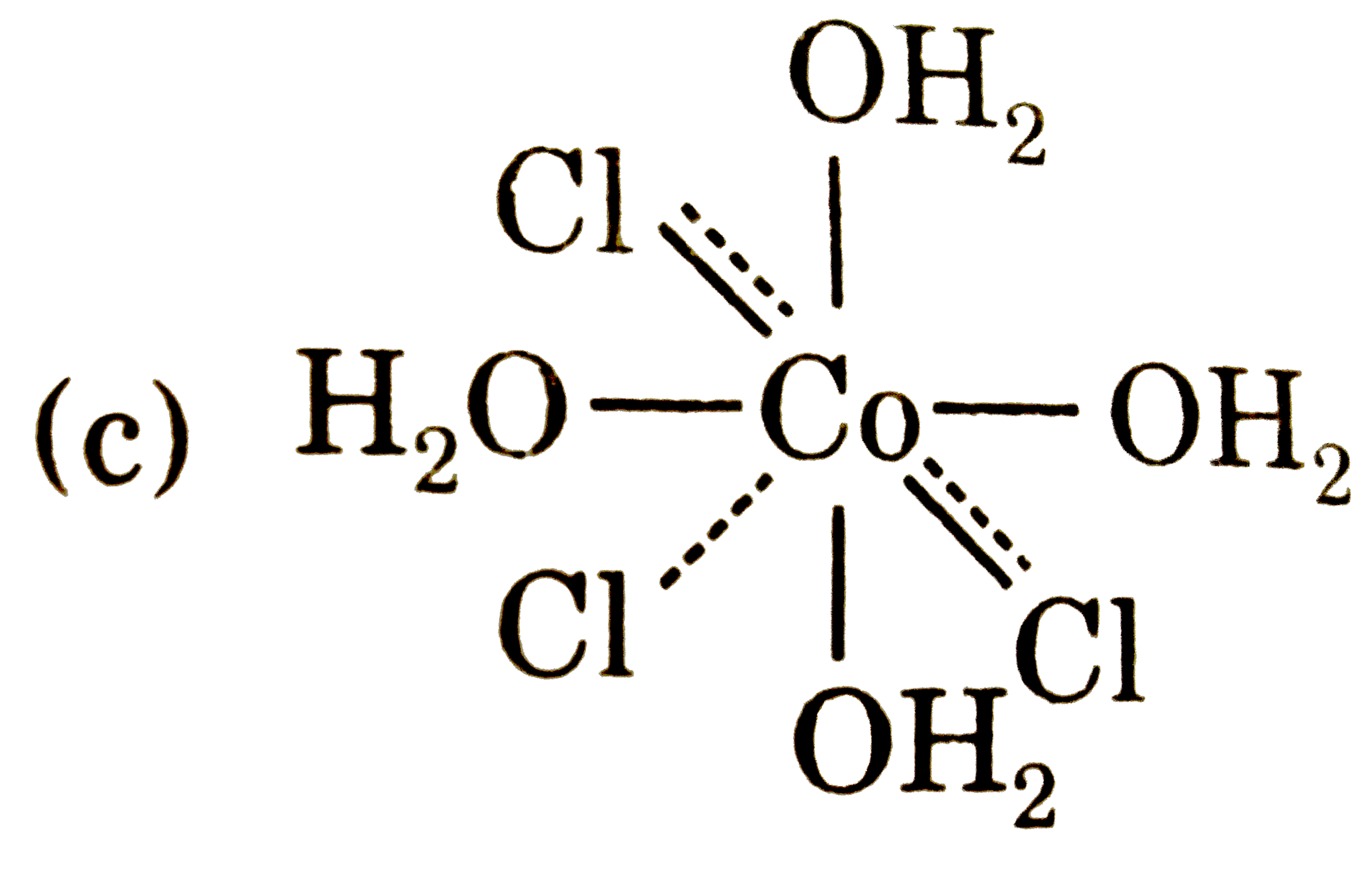A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise D.VBT, CFT, Hybridisation|123 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise E. Stability, Colour and Magnetic Properties of Complexes|60 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise B.Nomenclature of Coordination Compounds|29 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-C. Werners Theory, EAN Rule
- Whch of the following will not follow sidgwick's EAN rule ?
Text Solution
|
- The molecular formula Co(NH(3))(5)BrSo(4) have two isomeric form X and...
Text Solution
|
- CoCl(3).4H(2)O is an anyhydrous binary solute hence its Werner's repre...
Text Solution
|
- A complex of platinum, ammonia and chloride produces four ions per mol...
Text Solution
|
- How many moels of AgCI would be obtained, when 100 mL of 0.1 M CO(NH(3...
Text Solution
|
- One mole of complex compound Co(NH3)5Cl3 gives 3 moles of ions on diss...
Text Solution
|
- Complexes [Co(SO(4))(NH(3))(5)]Br and [CoBr(NH(3))(5)]SO(4) can be dis...
Text Solution
|
- Consider the following satements for Werner's theory : (P) Ligands a...
Text Solution
|
- Which of the following is correct for both the following coordination ...
Text Solution
|
- 50 ml of 0.2 M solution of a compound with empirical formula CoCl(3)....
Text Solution
|
- Which of the following is non-conducting ?
Text Solution
|
- Choose the complex entity which follow(s) EAN rule :
Text Solution
|
- A solution containing 0.319 g of complex CrCl(3).6H(2)O was passed th...
Text Solution
|
- Other than the X-ray difference , how could be the following pairs of ...
Text Solution
|
- The EAN of metal atoms in [Fe(CO)(2)(NO^(+))(2)] and Co(2)(CO)(8) resp...
Text Solution
|
- EAN of the elements (*) are equla in :
Text Solution
|
- Consider the following complexes: (I) K(2)Ptcl(6) (II) PtCl(4).2...
Text Solution
|
- Following Sidgwick's rule of EAN, Co(CO)(x) will be.
Text Solution
|
- Choose the correct option regarding the following complex compound whi...
Text Solution
|
- The effective atomic number of Rh (atomic number = 45) in the complex ...
Text Solution
|