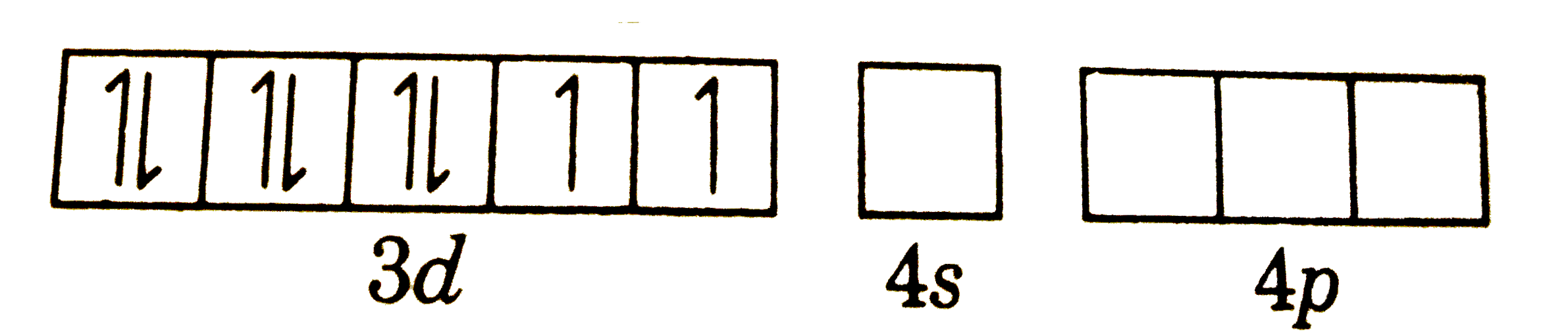
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise E. Stability, Colour and Magnetic Properties of Complexes|60 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise F.Isomerism in Coordination Compounds|124 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise C. Werners Theory, EAN Rule|63 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-D.VBT, CFT, Hybridisation
- For the correct assignment of electronic configuration of a complex, t...
Text Solution
|
- A complex of certain metal has the magnetic moment of 4.91 BM whereas ...
Text Solution
|
- Which of the following is correct for the complex [NiBr(2)(PPH(3))(2)]...
Text Solution
|
- Which of the following statement is not correct ? (a) [Ni(H(2)O)(6)...
Text Solution
|
- Both [Ni(CO)(4)] and [Ni(CN)(4)]^(2-) are diamagnetic. The hybridisati...
Text Solution
|
- Nickel (Z=28) combines with a uninegative monodentate ligand X^(-) to ...
Text Solution
|
- The complex [Fe(H(2)O)(5)NO]^(2+) is formed in the 'brown ring test' f...
Text Solution
|
- [Fe(en)(2)(H(2)O)(2)]^(2+) +en to " complex(X)". The correct statement...
Text Solution
|
- Which of the following complexes are low spin diamagnetic ? (P) K(4)...
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- For Co(II), (Choose incorrect statement):
Text Solution
|
- Which of the following are square planar complexes ? (P) [AuCl(4)]^(...
Text Solution
|
- Which of the following complexes is correctly matched with their geome...
Text Solution
|
- Which one of the following has a square planar geometry ? [At. no. Co=...
Text Solution
|
- S-1 : [Cr(NH(3))(6)]^(3+) is a inner orbital complex with S-2: The c...
Text Solution
|
- In which of the following octahedral complexes of Co (at. no. 27), wil...
Text Solution
|
- Among [Ni(CN)(4)]^(4-), [Ni(PPh(3))(3)Br] and [Ni(dmg)(2)] species, th...
Text Solution
|
- The value of 'spin only' magnetic moment for one of the following conf...
Text Solution
|
- Which of the following has dsp^(2) hybridization and is diamagnetic in...
Text Solution
|
- Which one is an outer orbital complex ?
Text Solution
|