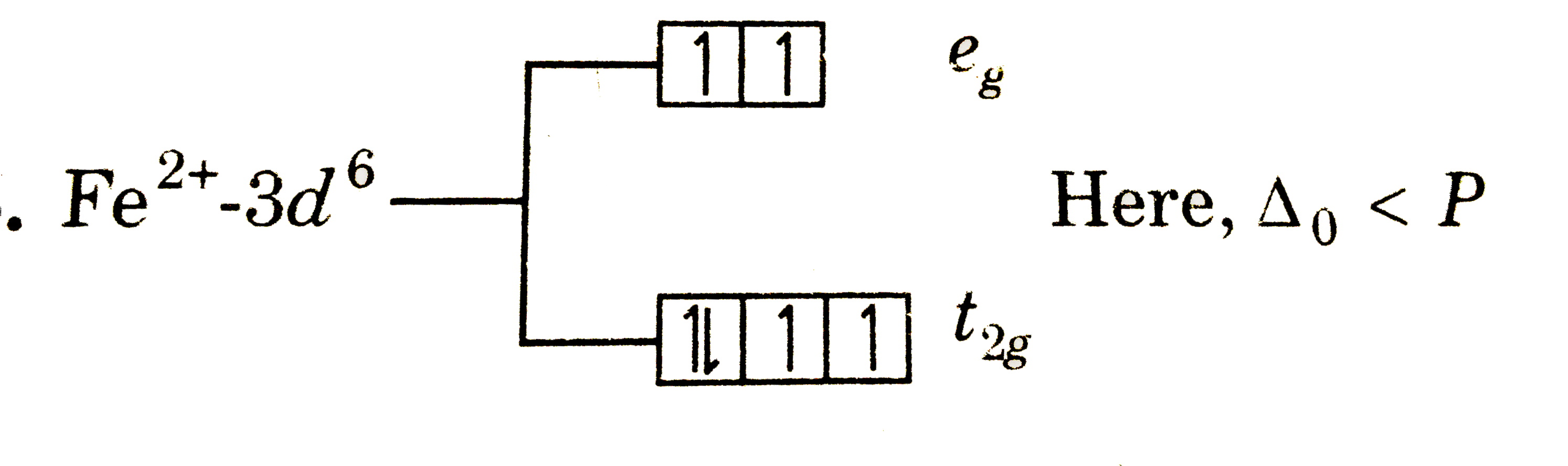A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise E. Stability, Colour and Magnetic Properties of Complexes|60 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise F.Isomerism in Coordination Compounds|124 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise C. Werners Theory, EAN Rule|63 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-D.VBT, CFT, Hybridisation
- The geometry of [Co(CO)(4)]^(-)" and "[Cd(CN)(4)]^(2-) are :
Text Solution
|
- In which option ,properties of the given three complexes are correct ?...
Text Solution
|
- If triangle(0) is crystal field splitting energy and P is mean pairing...
Text Solution
|
- Which of the following is inner orbital complex as well as diamagnetic...
Text Solution
|
- Consider the following complexes : [NiCl(4)]^(2-), [Ni(CN)(4)]^(2-),...
Text Solution
|
- [Ni(CN)(4)]^(2-)" and "[NiCl(4)]^(2-) have similarity but not in :
Text Solution
|
- For which of the following d^(n) configuration of octahedral complex(e...
Text Solution
|
- The correct order of energies of d-orbitals of metal ion in a square p...
Text Solution
|
- According to crystal field theory (CFT) :
Text Solution
|
- Which amongst following is called spin paired complex ?
Text Solution
|
- Which of the following complexes are tetrahedral but spin free ?
Text Solution
|
- Which of the following is not a shortcoming of VBT ? (P) Quantitativ...
Text Solution
|
- If crystal field theory is completely followed, then which of the foll...
Text Solution
|
- Ruby is aluminium oxide (Al(2)O(3)) containing about 0.5-1% Cr^(3+) io...
Text Solution
|
- For [FeF(6)]^(3-) and [CoF(6)]^(3-), the statement that is correct is ...
Text Solution
|
- Which of the following complex ions has electrons that are symmetrical...
Text Solution
|
- Which of the following statement is false ?
Text Solution
|
- The number of unpaired electrons in d^(6), low spin, octahedral comple...
Text Solution
|
- Which of the following option is correct for [Fe(H(2)O)(6)]^(2+)" and ...
Text Solution
|
- Which of the following complex does not have tetrahedral geometry ?
Text Solution
|