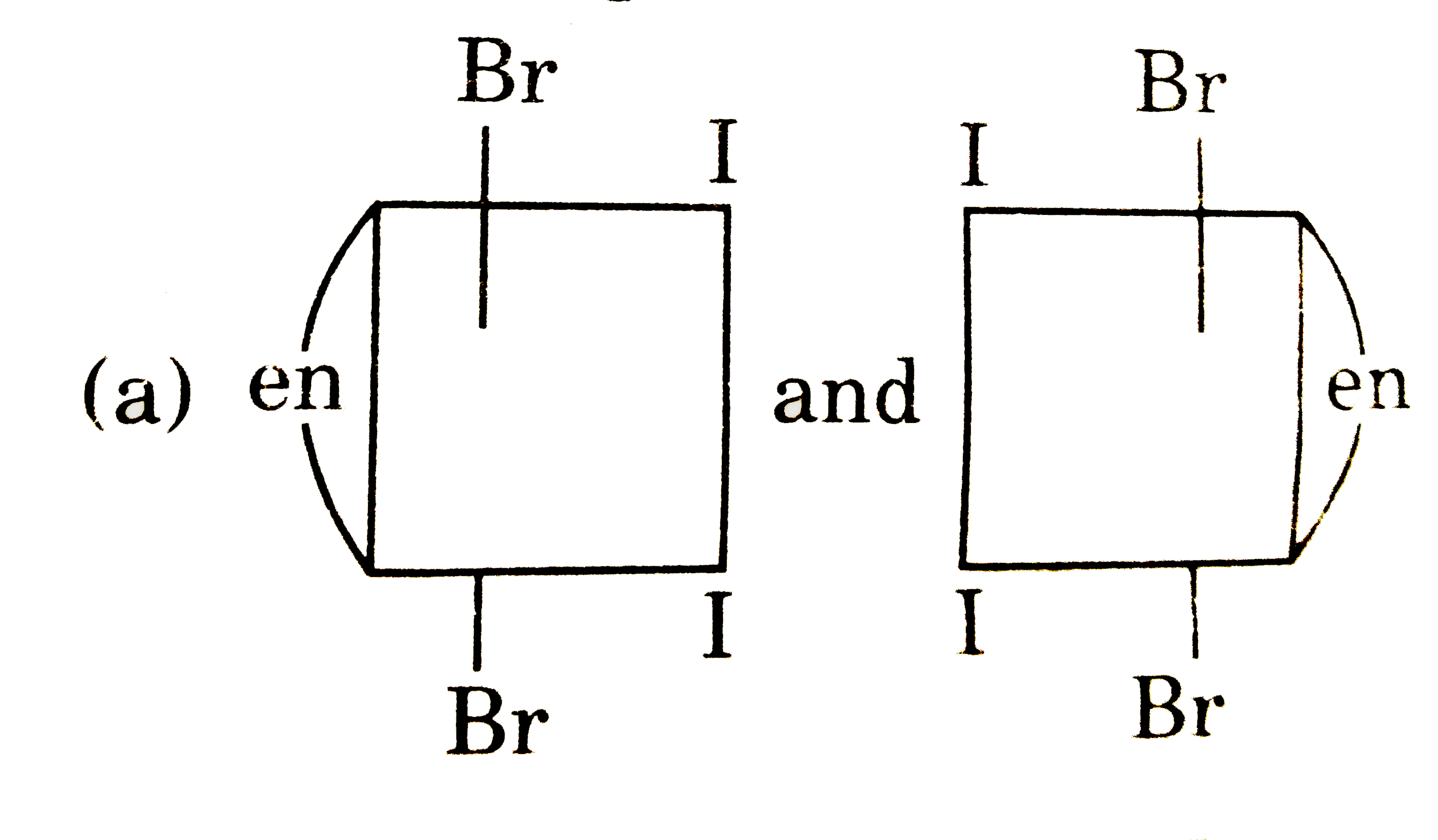
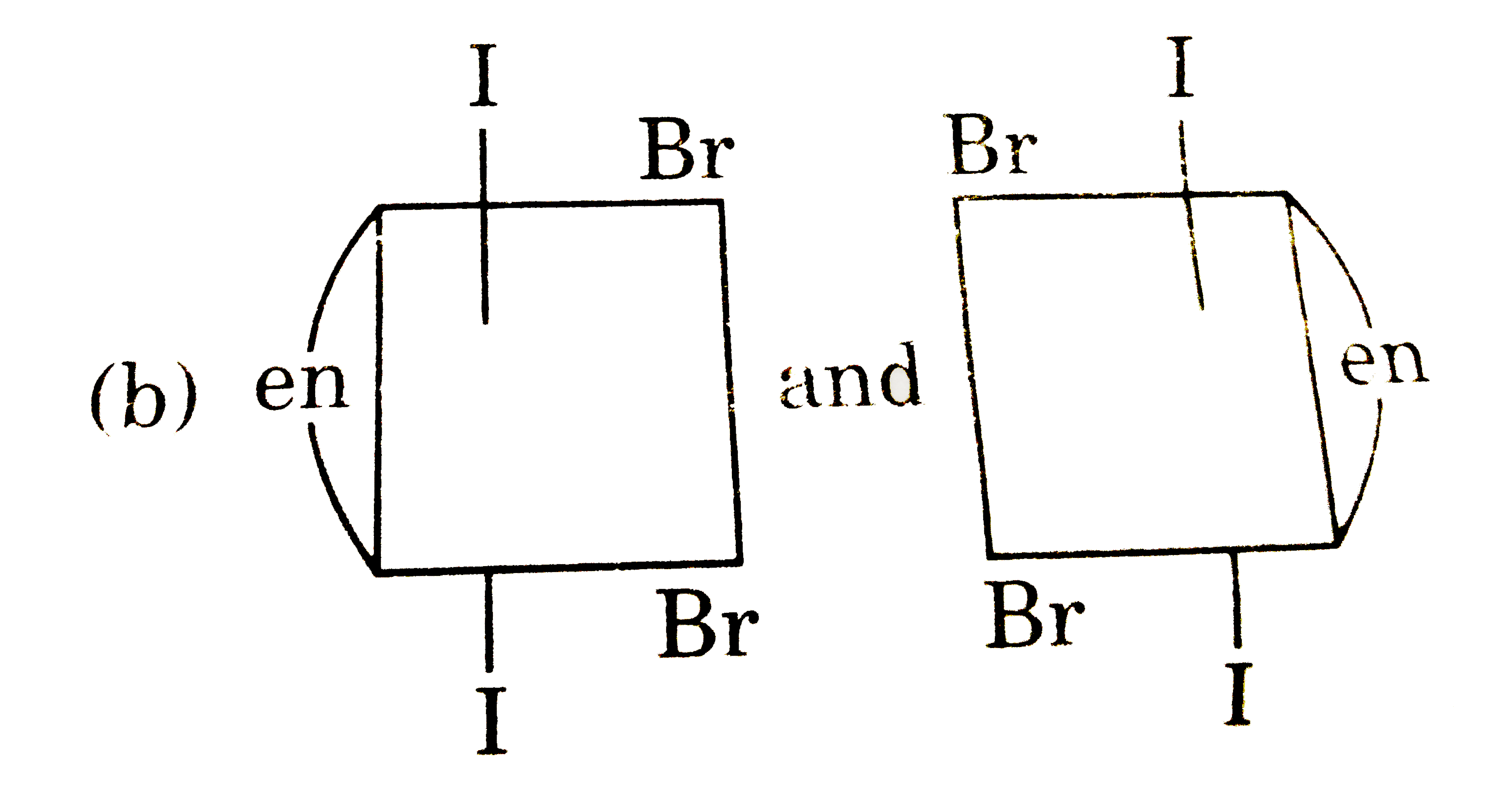
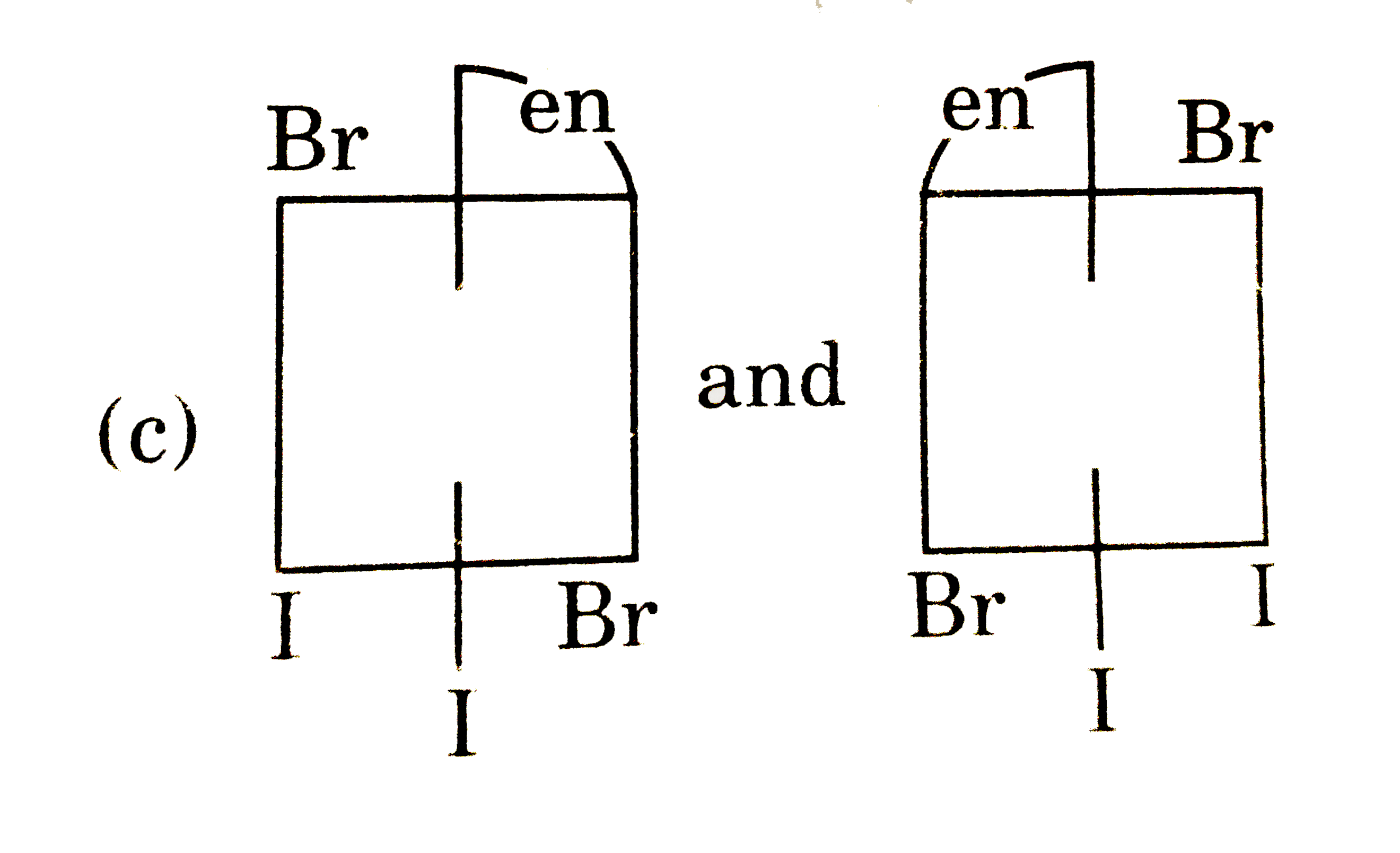
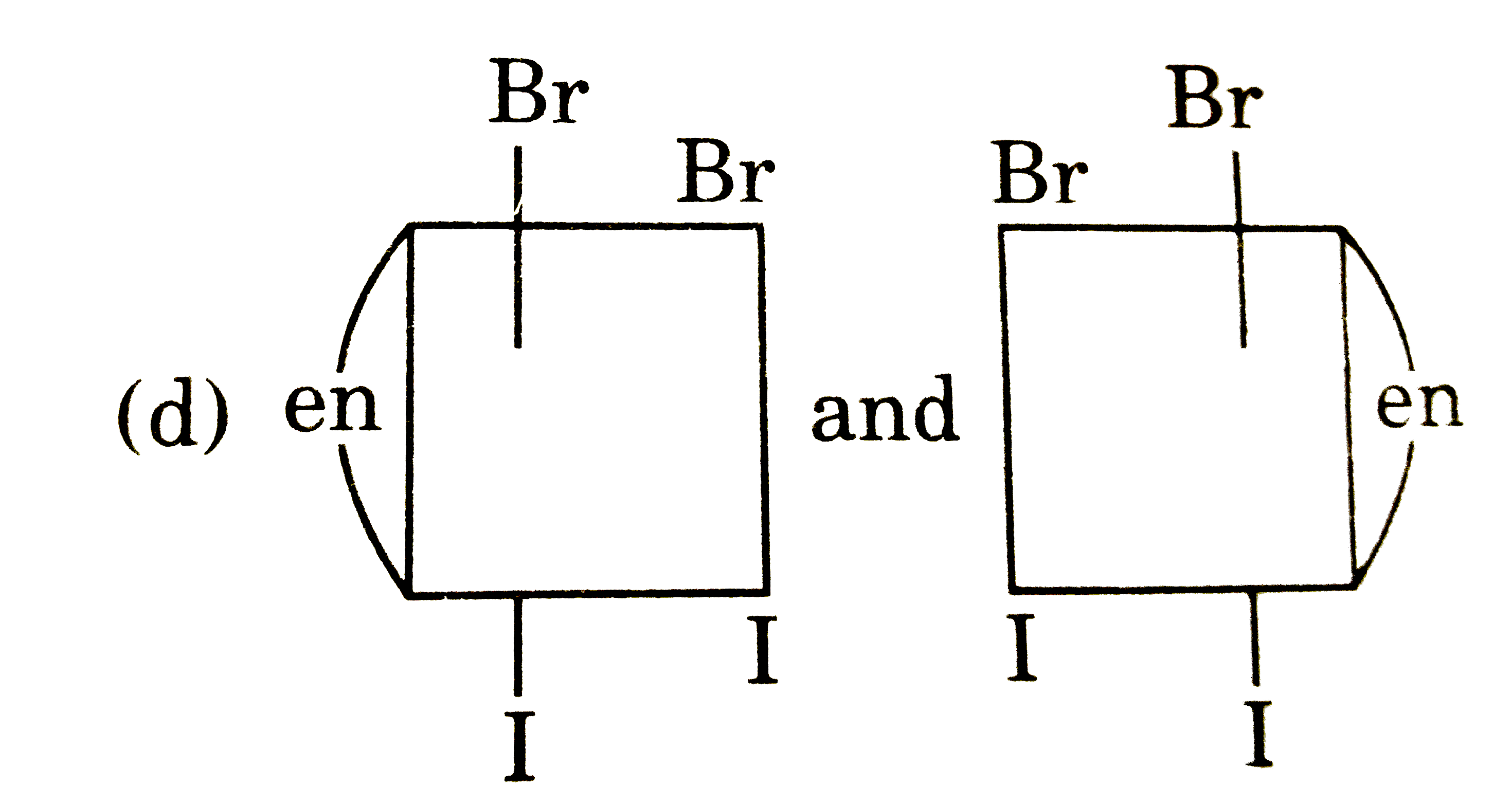
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise G. Organometallic Compounds, Synergic Bonding|52 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise REASONING TYPE|59 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise E. Stability, Colour and Magnetic Properties of Complexes|60 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-F.Isomerism in Coordination Compounds
- [Co(en)3]^(3+) ion is expected to show
Text Solution
|
- Which of the following complex will show optical activity ?
Text Solution
|
- The complex ion . [M(en)Br(2)I(2)]^(-1), has two optical isomers. Thei...
Text Solution
|
- Consider the following statements and select the correct option using ...
Text Solution
|
- Which of the following has an optical isomer?
Text Solution
|
- Which of the following pairs of structure shows geometrical isomerism ...
Text Solution
|
- Which of the following pairs represents linkage isomers?
Text Solution
|
- Select the correct statement regarding [Pt(CH(3)-CH(NH(2))COO)Cl(2)]^(...
Text Solution
|
- The diamagnetic complex which has two geometrical and 4 optically acti...
Text Solution
|
- The compexes [Co(NH(3))(6)][Cr(C(2)O(4))(3)] and [Cr(NH(3))(6)][Co(C(2...
Text Solution
|
- Which of the following compounds show optical isomerism? I. cis -...
Text Solution
|
- Which one has largest number of isomers ?
Text Solution
|
- Which of the following statement is incorrect ?
Text Solution
|
- Which of the following statements is not true about the complex ion [P...
Text Solution
|
- Of the following complex ions, one exhibits isomerism. That is :
Text Solution
|
- Which of the following will be able to show geometrical isomerism ?
Text Solution
|
- The number of geometrical isomers for octahedral [Co(NH(3))(2)Cl(4)]^(...
Text Solution
|
- Select the correct statement from the following :
Text Solution
|
- Which of the following isomers of [M(NH(3))(2)Cl(2)] would react with ...
Text Solution
|
- Which of the following is correct regarding [Pt(NH(3))(2)(CN)(2)] ?
Text Solution
|