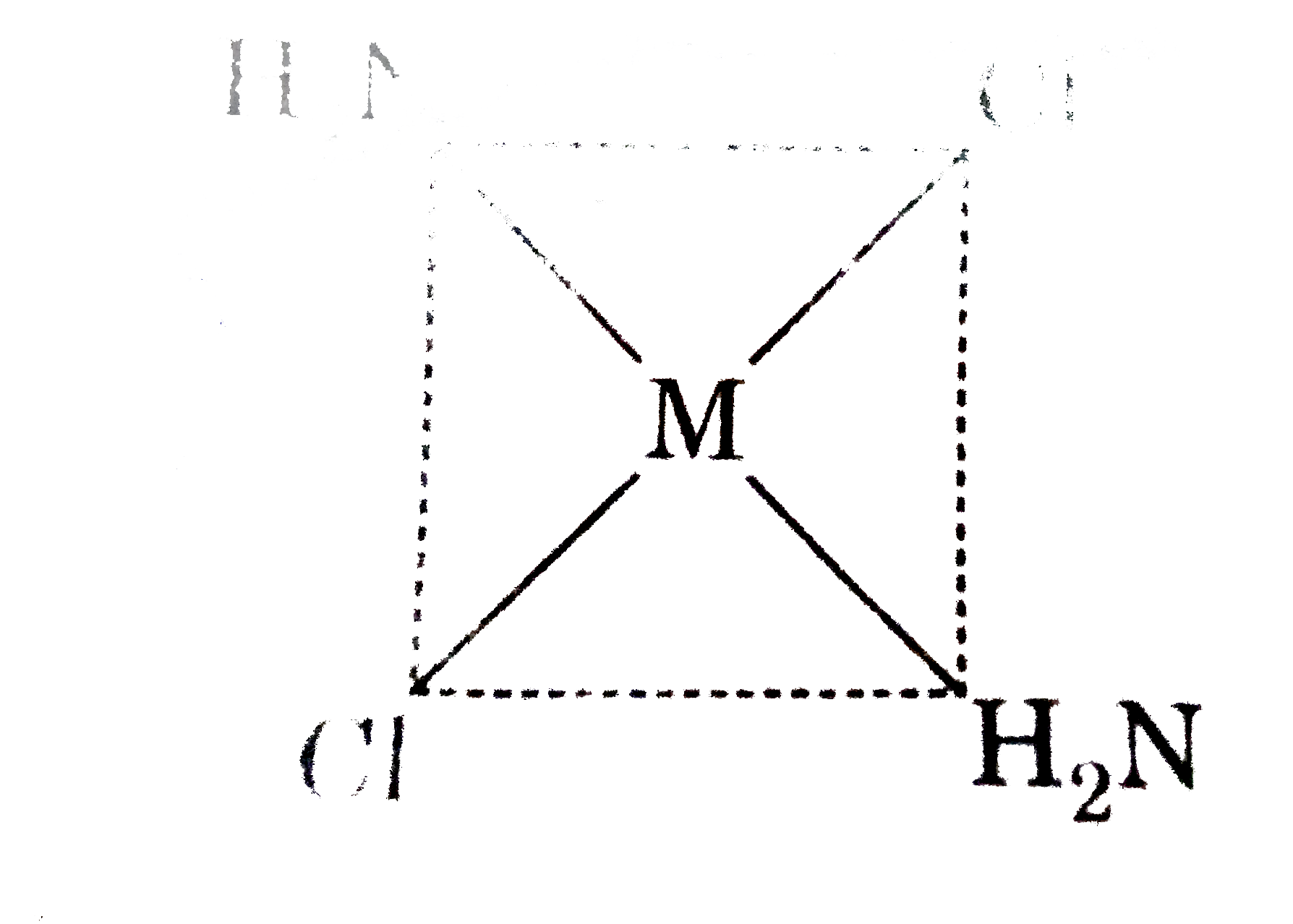
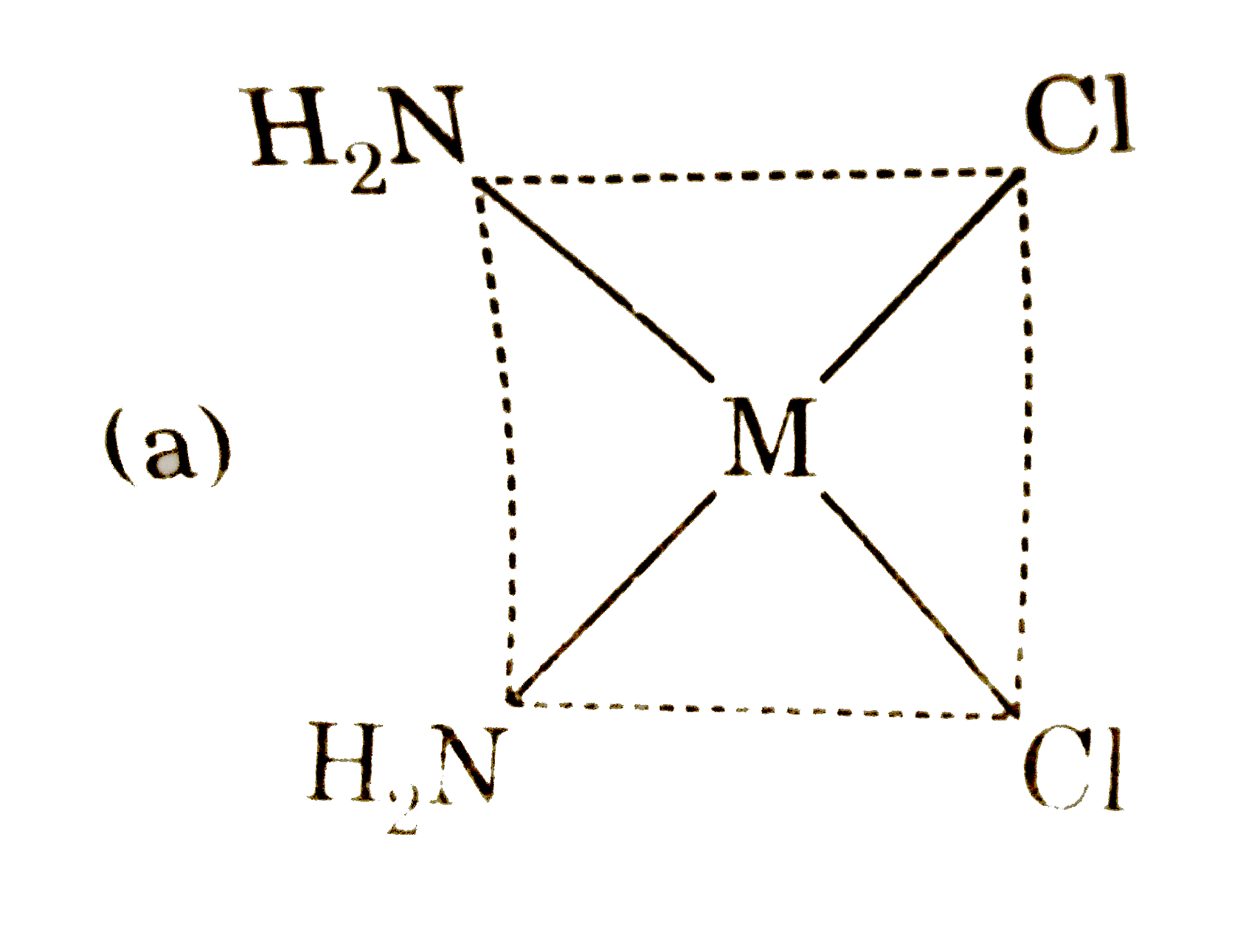A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise G. Organometallic Compounds, Synergic Bonding|52 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise REASONING TYPE|59 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise E. Stability, Colour and Magnetic Properties of Complexes|60 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-F.Isomerism in Coordination Compounds
- The number of geometrical isomers for octahedral [Co(NH(3))(2)Cl(4)]^(...
Text Solution
|
- Select the correct statement from the following :
Text Solution
|
- Which of the following isomers of [M(NH(3))(2)Cl(2)] would react with ...
Text Solution
|
- Which of the following is correct regarding [Pt(NH(3))(2)(CN)(2)] ?
Text Solution
|
- The complex which exhibits geometrical as well as optical isomerism is...
Text Solution
|
- How many isomers possible for [Co(gly)(dmg)(en)]^(+1)?
Text Solution
|
- Which of the following does not show fac-mer isomerism ?
Text Solution
|
- Which of the following statements are true ?
Text Solution
|
- How many geometrical isomers and stereoisomers are possible for [Pt(NO...
Text Solution
|
- The two compounds pentaamminesulphatocobalt (III)bromide and pentaammi...
Text Solution
|
- How many total possible isomers are present in the following complex ?...
Text Solution
|
- Which of the following will exhibit geometrical as well as optical iso...
Text Solution
|
- How many total possible isomers are present in the following complex ?...
Text Solution
|
- Total possible linkage isomers of K(4)[Fe(CN)(5)(NO(2))] is :
Text Solution
|
- How many geometrical isomers are possible for [Pd^(2+)(NH(2)-CH(CH(3))...
Text Solution
|
- [Cr(NH(3))(5)Br]Cl and [Cr(NH(3))(5)Cl]Br can be distinguished by/and ...
Text Solution
|
- Which kind of isomerism is exhibited by [Co(EDTA)]-
Text Solution
|
- Which one of the following statements are correct for the complex [Co(...
Text Solution
|
- Which of the following statements are true ?
Text Solution
|
- If a complex, Ma(4)b(2) (where M is a central atom and a and b are mon...
Text Solution
|