A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise G. Organometallic Compounds, Synergic Bonding|52 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise REASONING TYPE|59 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise E. Stability, Colour and Magnetic Properties of Complexes|60 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-F.Isomerism in Coordination Compounds
- Due to the presence of ambidenate ligands coordination compounds show ...
Text Solution
|
- Four statements for the following reaction given below [CoCl(2)(NH(3))...
Text Solution
|
- The complex that shows optical activity is :
Text Solution
|
- The correct statement on the isomerism associated with the follow...
Text Solution
|
- In coordination compounds , the hydrate isomers differ in .
Text Solution
|
- The phenomenon of optical activity will be shown by:
Text Solution
|
- Find the pair of compounds in which both show geometrical as well as o...
Text Solution
|
- Two complex compounds with same representation [CoCl(2)(NH(3))(4)]Cl h...
Text Solution
|
- In which of the following pairs both the complex show optical isomeris...
Text Solution
|
- which of the following complexes show ionization isomerism ?
Text Solution
|
- The number of geometrical isomers of [Co(NH(3))(3)(NO(3))(3)] is
Text Solution
|
- How many different isomers exist for the octahedral complex [Co(NH(3))...
Text Solution
|
- How many isomers exist for the octahedral compound, Pt(NH(3))(2)Cl(4)?
Text Solution
|
- How many isomers exist for a square planar platinum compound which has...
Text Solution
|
- How many isomers of octahedral Co(NH(3))(3)Cl(3) are there ?
Text Solution
|
- How many isomers exist for the [Co(NH(3))(4)Cl(2)]^(+)" and "[Co(en)(2...
Text Solution
|
- Which of the following complex is incorrectly matched against their in...
Text Solution
|
- Which of the following complex will have three isomeric forms ?
Text Solution
|
- Total number of geometrical isomers of [M(AB)c(2)d(2)]:
Text Solution
|
- Number of geometrical isomer(s) of square planar complex [RhCl(PPh(3))...
Text Solution
|
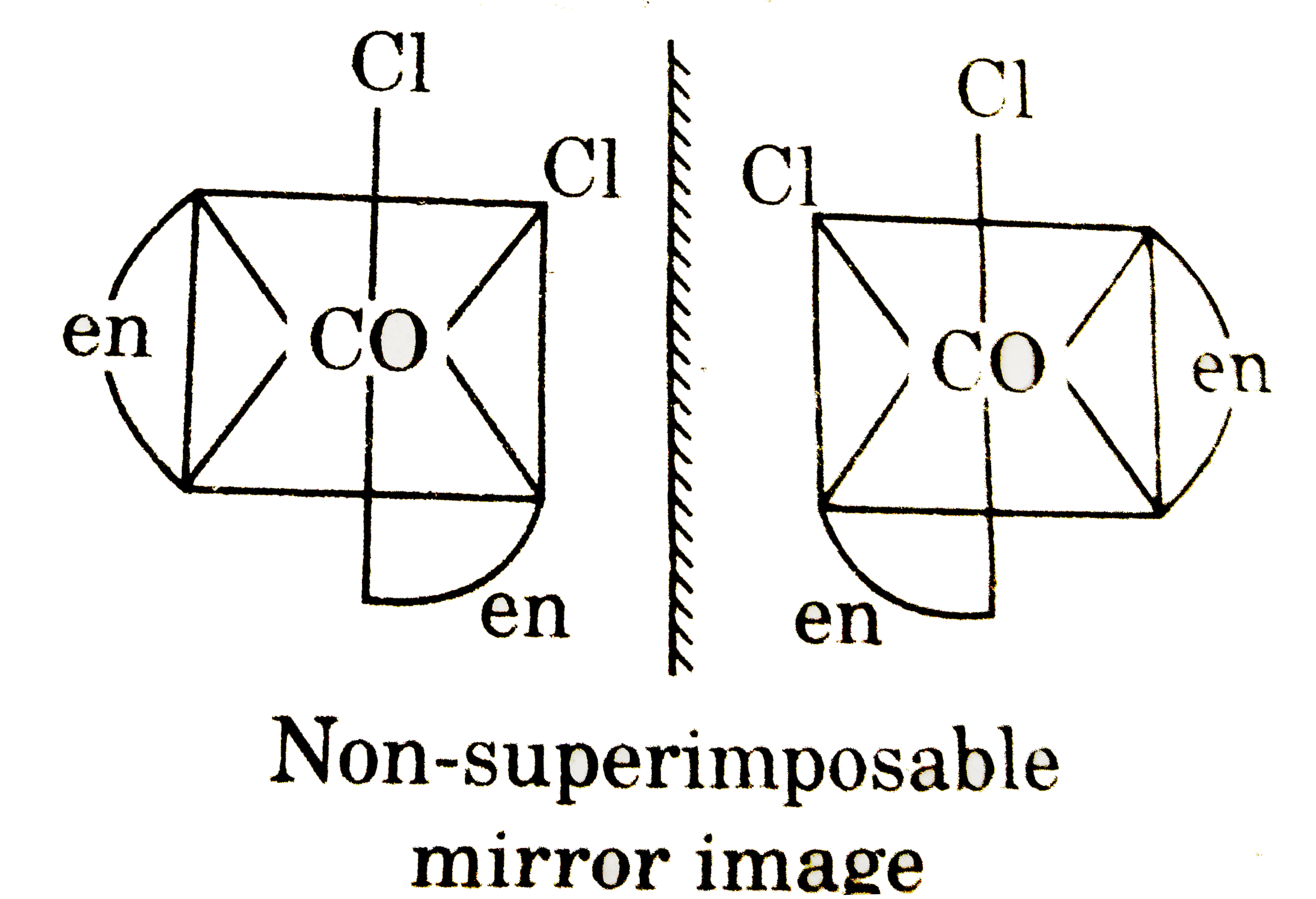 Non-superimposable mirror image
Non-superimposable mirror image