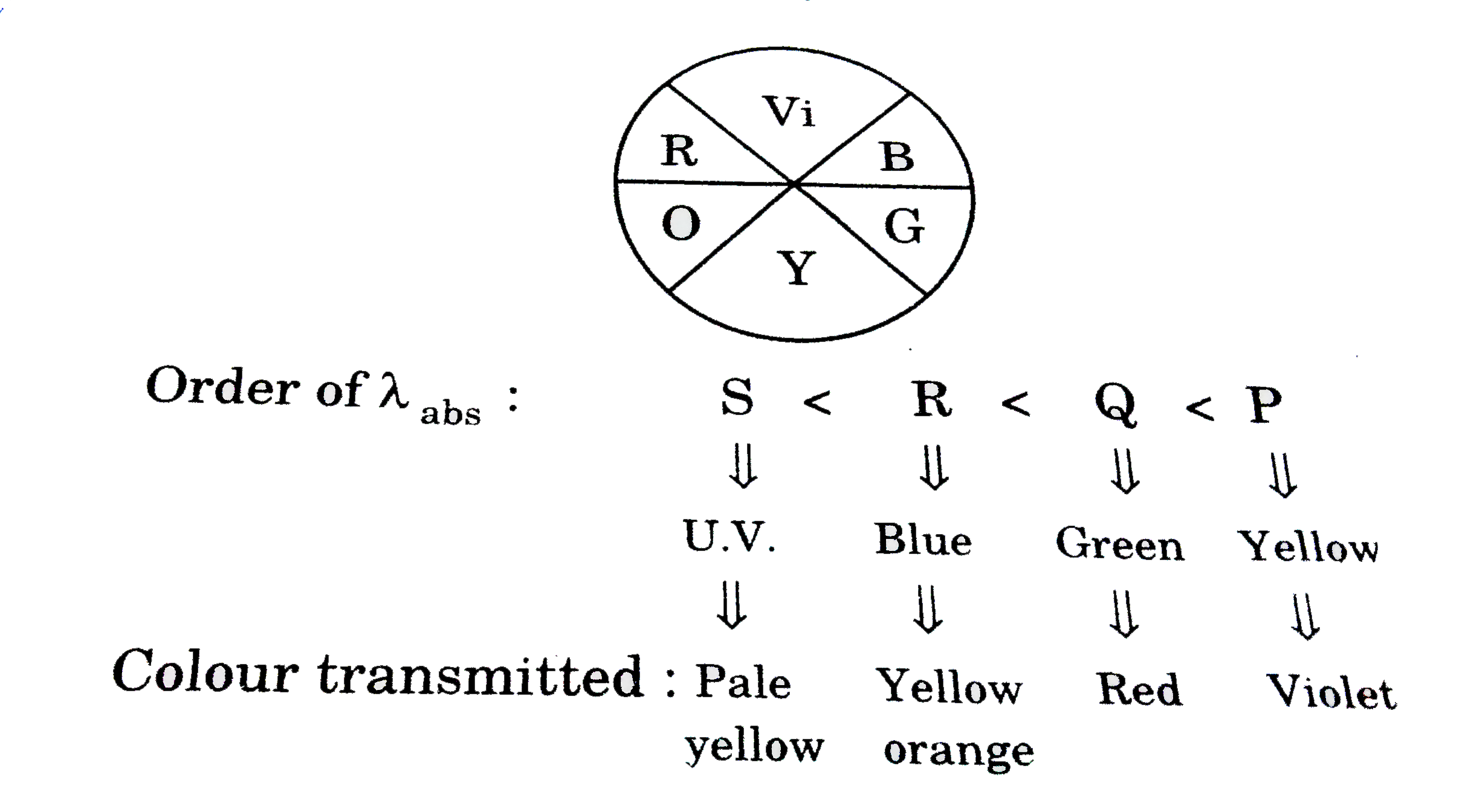A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
GRB PUBLICATION|Exercise COMPREHENSION 1|3 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise COMPREHENSION 2|3 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise REASONING TYPE|59 VideosCHEMICAL KINETICS
GRB PUBLICATION|Exercise Subjective Type|63 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-MULTIPLE OBJECTIVE TYPE QUESTION
- Which of the following ions can form paramagnetic as well as diamagnet...
Text Solution
|
- Find the number of unpaired electrons in each case [Mn(CN)(6)]^(3-)=...
Text Solution
|
- Four complexes are given : (P) [CoCl(NH(3))(5)]^(2+)" "(Q) [Co(NH(...
Text Solution
|
- In which of the following, the central metal atom is behaving as a lew...
Text Solution
|
- Choose the correct statement(s) :
Text Solution
|
- In Pt(C(2)H(4))(2)(C(2)F(4))(2) molecule, in which the platinum and al...
Text Solution
|
- Which of the chelating ligands maintain hapticity equal to denticity ?
Text Solution
|
- Consider following 4 complexes : (P) [Cr(CO)(5)(Pet(3))]" "(Q) [Cr...
Text Solution
|
- VBT model for [MnCo(CO)(9)] suggests :
Text Solution
|
- Which of the following are paramagnetic ?
Text Solution
|
- Which of the following can show coordination isomerism ?
Text Solution
|
- Which is/are correct statement(s)?
Text Solution
|
- Atomic number of Mn. Fe and Co are 25, 26 and 27 respectively. Which o...
Text Solution
|
- Atomic number of Mn, Fe, Co and Ni are 25, 26, 27 and 28 respectively....
Text Solution
|
- Which of the following options are correct for [Fe(CN)(6)]^(3-) comple...
Text Solution
|
- An aqueous pink solution of cobalt (II) chloride changes to deep blue ...
Text Solution
|
- Which of the following complexes are homoleptic ?
Text Solution
|
- Which of the following complexes are heteroleptic ?
Text Solution
|
- Identify the optically active compounds from the following :
Text Solution
|
- Identify the correct statements for the behaviour of ethane-1, 2-diami...
Text Solution
|