Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-CHEMICAL BONDING-I-Subjective Type
- Find the sum of triangular BO(3) unit and BO(4) tetrahedral unit in bo...
Text Solution
|
- Total number of CN^(-) ion that are present in ICN(liq.) {Hint : ICN(l...
Text Solution
|
- The dipole moment of HBr is 2.6 xx 10^(-30)Cm and interatomic spacing ...
Text Solution
|
- Find out the total number of species containing, E-E(N-N/P-P) linkage....
Text Solution
|
- The number of water molecule(s) directly bonded to the metal centre in...
Text Solution
|
- Based on VSEPR theory, the number of 90 degree F-Br-F angles in BrF(5)...
Text Solution
|
- Number of peroxy linkage in a H(2)S(2)O(8) (Marshall acid) molecule is...
Text Solution
|
- Answer the following questons about the strucute of the dimer of phosp...
Text Solution
|
- Give the number of characteristic bond(s) found in the various oxy-aci...
Text Solution
|
- For a given molecule : CF(2)=C=C=C=CH-CH(3) ltbrrgt find the maximum...
Text Solution
|
- How many cidic hydrogen is/are present in CsH(2)PO(2)?
Text Solution
|
- underset(2(X)overset(-H(2)O)rarr(Y)overset(+[O])rarr(Z)" ...
Text Solution
|
- Two molecules of oil of vitriol (ic-acid of sulphur) -H(2)O+Orarr(X). ...
Text Solution
|
- Find the number of sigma bond and pi bonds present in Marshall's acid...
Text Solution
|
- The number of species which consists of sp^(3)d hybridised central ato...
Text Solution
|
- The number of molecules is/are pyramidal in shape : XeO(3),NCl(3),Cl...
Text Solution
|
- Find the difference of the oxidation state of S-atoms in sodium pyrosu...
Text Solution
|
- Among the following, find the number of acid(s) which are having hypo ...
Text Solution
|
- P(4)O(10)overset(2H(2)O)rarrH(4)P(4)O(12)overset(H(2)O)rarrH(6)P(4)O(1...
Text Solution
|
- Find the number of dibasic oxyacids in the following: H(2)CO(3),H(2)...
Text Solution
|
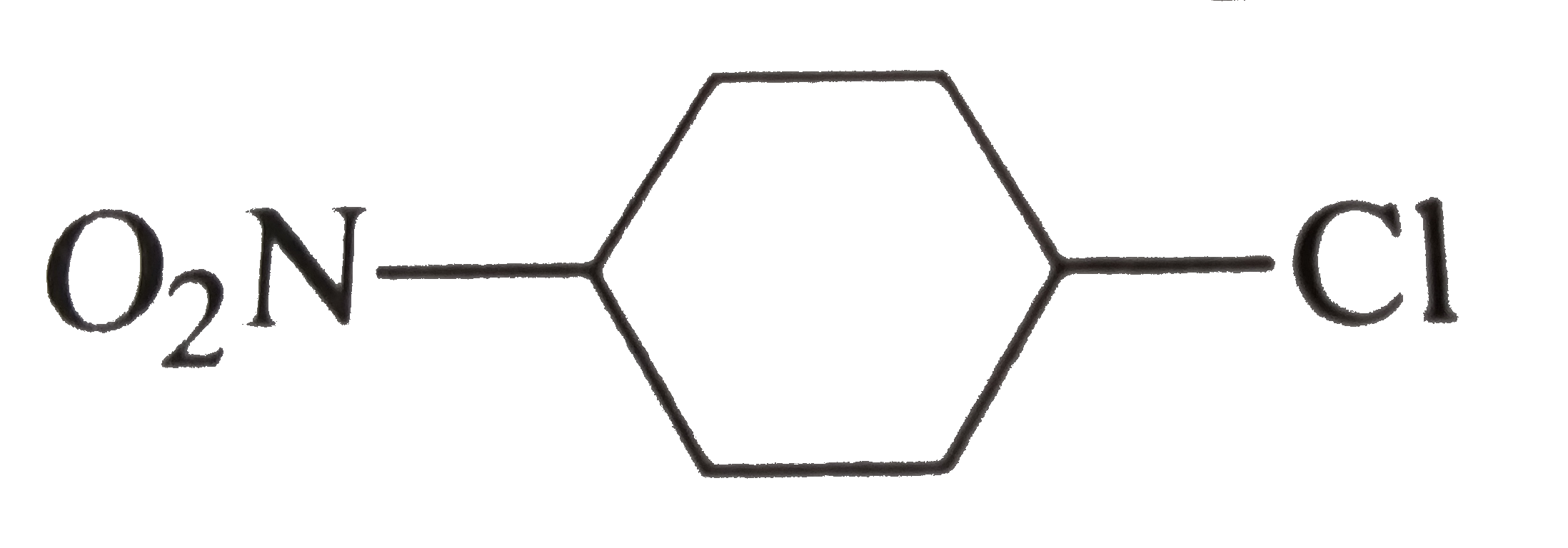 .
.