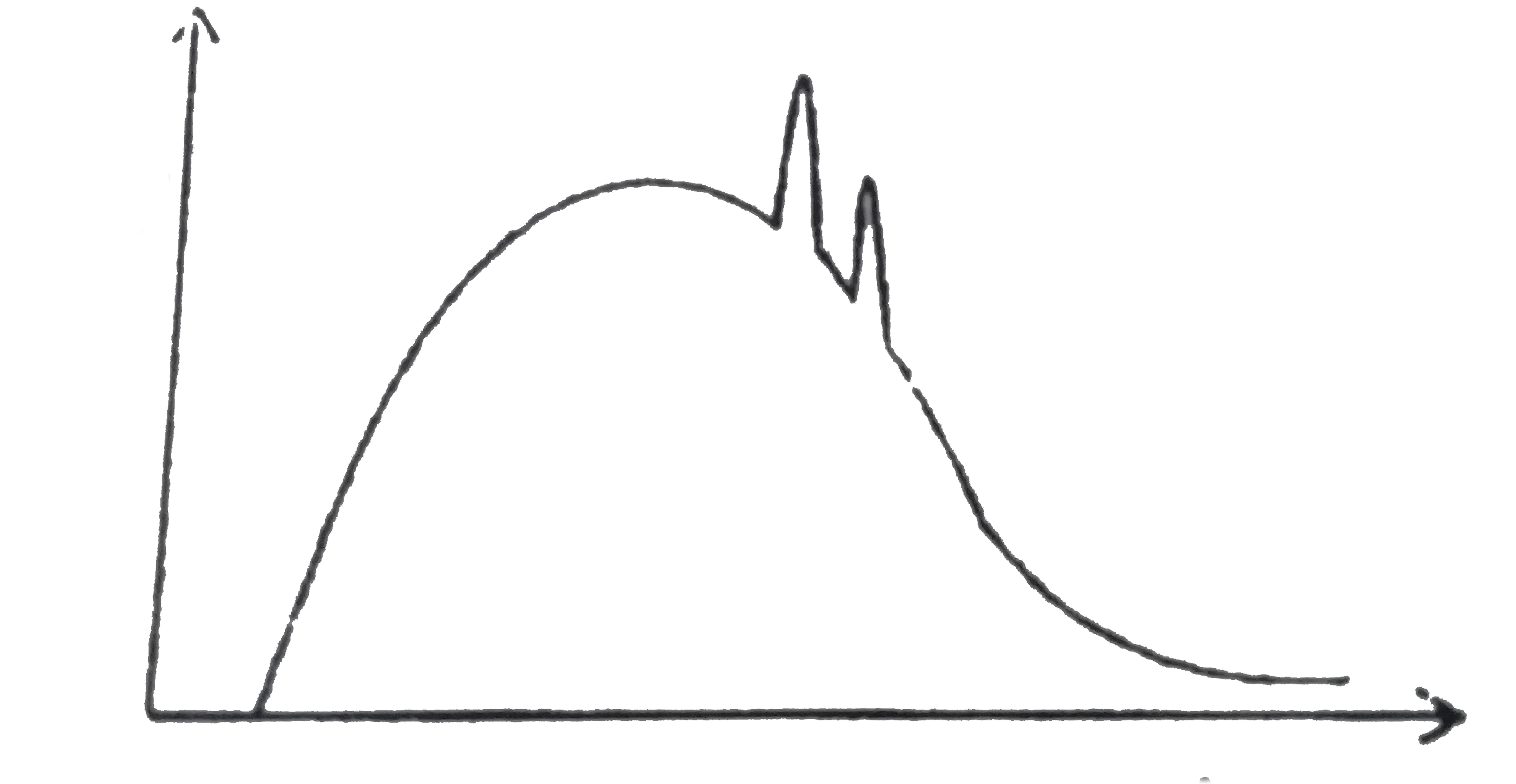
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A beam of electrons striking a cooper target produces X-rays. Its spec...
Text Solution
|
- When a beam of accelerated electrons hits a target , a continuous X - ...
Text Solution
|
- An electron beam is acceleration by a potential difference V to hit a ...
Text Solution
|
- A cobalt (atomic no. =27 ) target is bombereded with electrons and th...
Text Solution
|
- A beam of electrons striking a copper target produces X-rays. Its spec...
Text Solution
|
- The kinetic energy of electrons that strike the target is increased , ...
Text Solution
|
- A beam of 350 keV electrons a molybdenum target, generating the X-rays...
Text Solution
|
- An electron beam hits a target to produce continuous X-ray spectrum. I...
Text Solution
|
- X-ray are produced by accelerating electrons across a given potential ...
Text Solution
|