Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
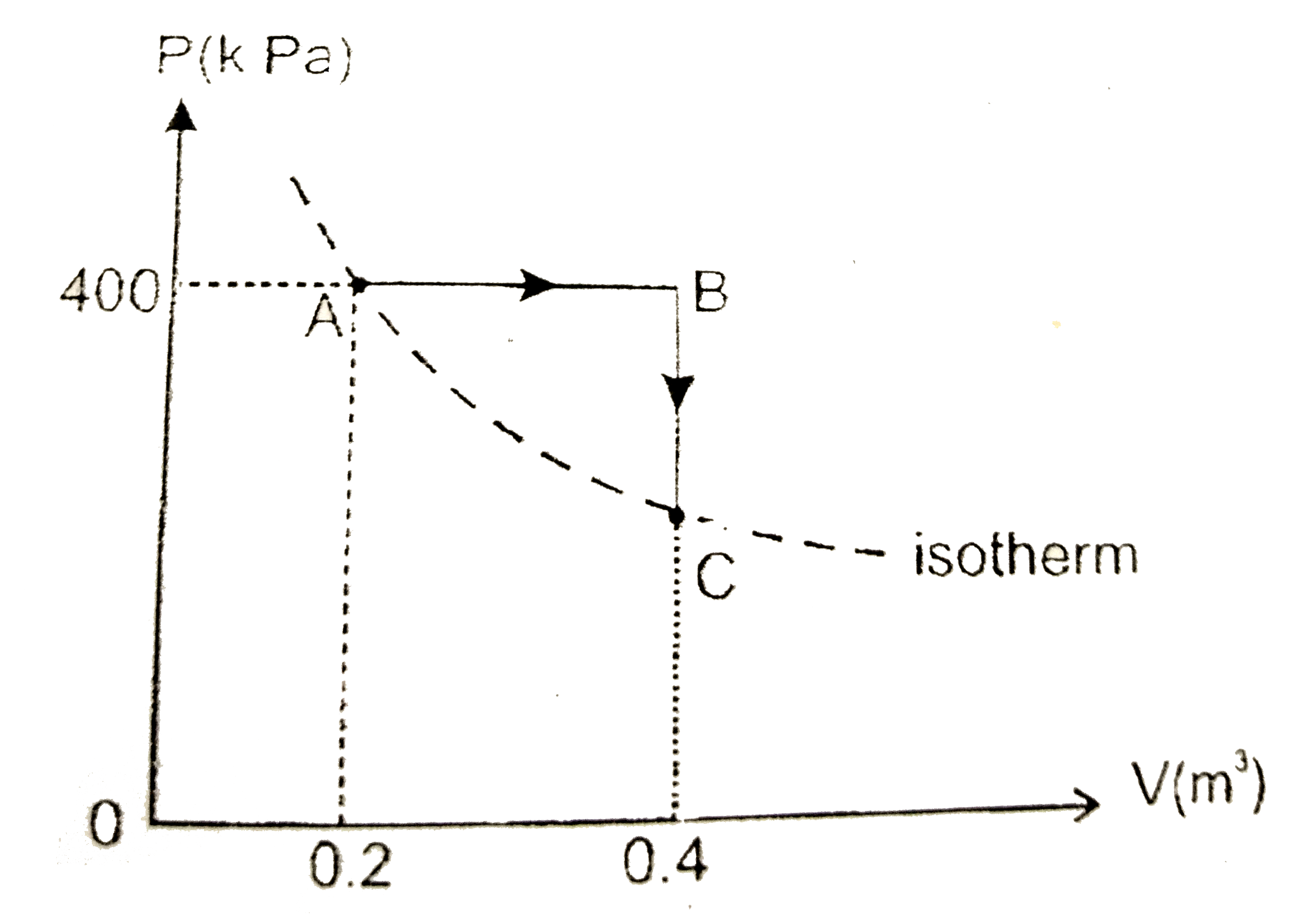
- An ideal gas undergoes a process ArarrBrarrC shown in P-V graph. Then ...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- One mole of a monoatomic ideal gas undergoes the process ArarrB in the...
Text Solution
|
- V-T graph of a process of monoatomic ideal gas is shown in figure. Hea...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- An ideal gas undergoes a process ArarrBrarrC shown in P-V graph. Then ...
Text Solution
|
- An ideal gas with heat capacity at constant volume C(V) undergoes a qu...
Text Solution
|