A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS -THERMODYNAMICS-All Questions
- Four curves A, B, C and D are drawn in Fig. for a given amount of gas....
Text Solution
|
- In the following pressure-volume diagram, the isochoric, isothermal an...
Text Solution
|
- The P-V diagram of a system undergoing thermodynnaic transformation is...
Text Solution
|
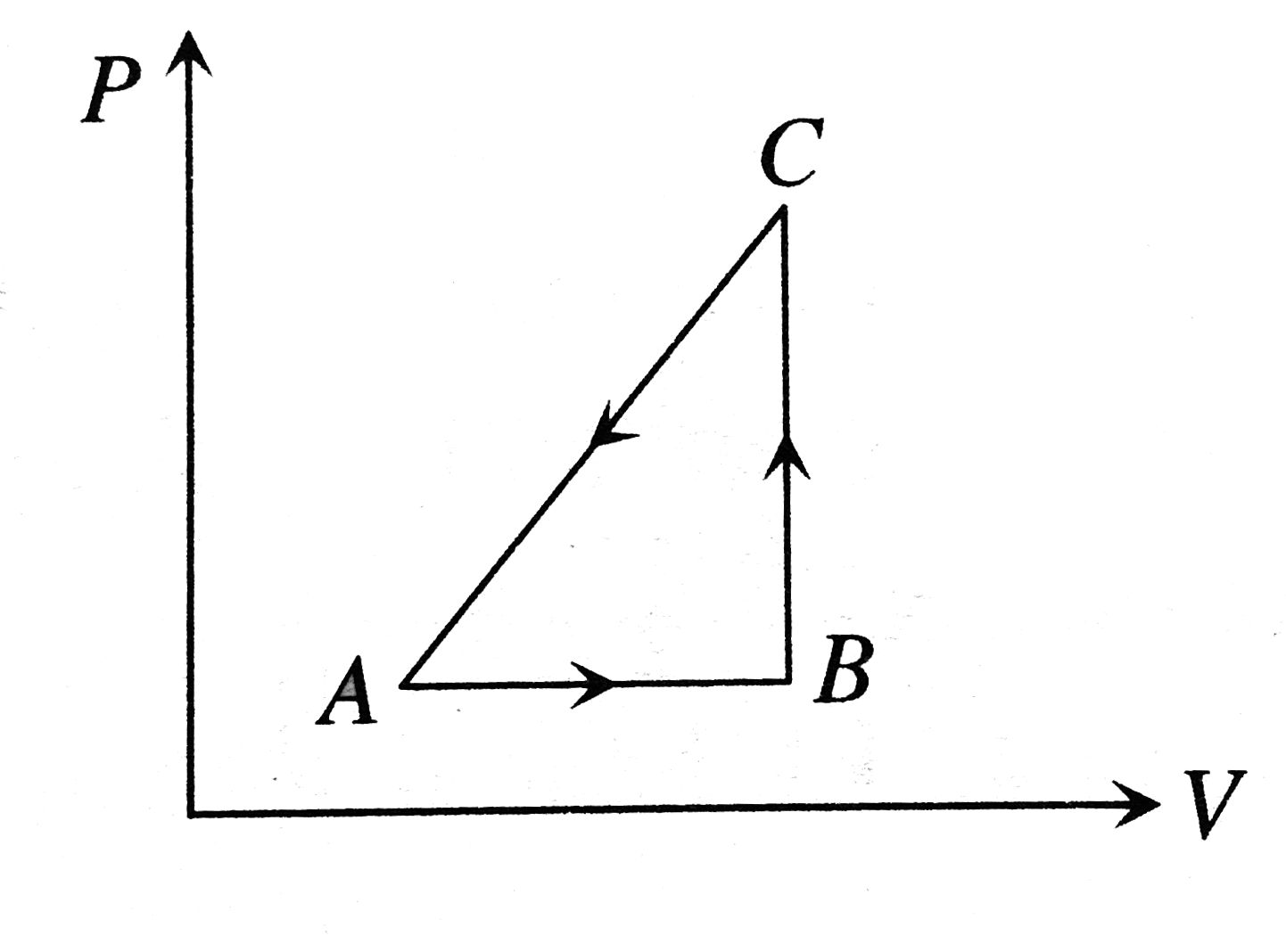
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
- In the following indicator diagram, the net amount of work done will b...
Text Solution
|
- The cyclic process for 1 mole of an ideal gas is shown in the V-T diag...
Text Solution
|
- A cyclic process ABCD is shown in the figure P - V diagram. Which of t...
Text Solution
|
- Carnot cycle (reversible) of a gas represented by a pressure volume cu...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|
- Work done in the given P - V diagram in the cyclic process is
Text Solution
|
- A cyclic process ABCA is shown in the V-T diagram process on the P-V
Text Solution
|
- In the figure given two processes A and B are shown by which a thermod...
Text Solution
|
- In the cyclic process shown in the figure, the work done by the gas in...
Text Solution
|
- An ideal gas is taken around the cycle ABCA shown in P - V diagram. Th...
Text Solution
|
- Heat energy absorbed by a system in going through a cyclic process sho...
Text Solution
|
- A thermodynamic system is taken from state A to B along ACB and is bro...
Text Solution
|
- A system is taken through a cyclic process represented by a circle as ...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- The P - V graph of an ideal gas cycle is shown here as below. The adia...
Text Solution
|
- An ideal monoatomic gas is taken the cycle ABCDA as shown in following...
Text Solution
|