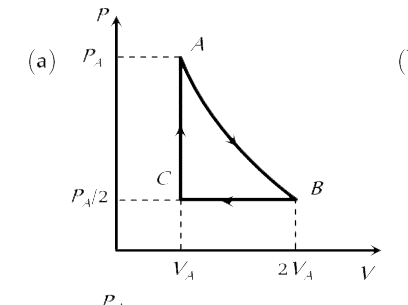
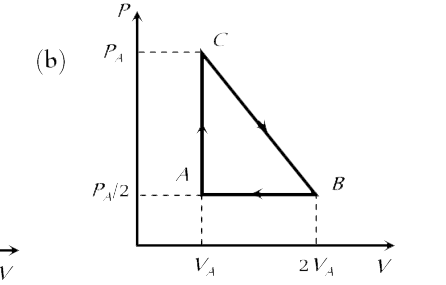
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS -THERMODYNAMICS-All Questions
- If a Carnot’s engine functions at source temperature 127^(@)C and at s...
Text Solution
|
- In the case of diatomic gas, the heat given at constant pressure is th...
Text Solution
|
- An ideal monoatomic gas is taken round the cycle ABCD A shown in the ...
Text Solution
|
- A gas is compressed adiabatically till its temperature is doubled. The...
Text Solution
|
- A tyre filled with air,(27^(@)C,o and 2 atm ) bursts, then what is tem...
Text Solution
|
- A gas expands adiabatically at constant pressure such that its tempera...
Text Solution
|
- P - V diagram of an ideal gas is as shown in figure. Work done by the ...
Text Solution
|
- An engineer claims to have made an engine delivering 10 kW power with ...
Text Solution
|
- An ideal gas heat engine operates in a Carnot cycle between 27^(@)C an...
Text Solution
|
- A gas expands with temperature according to the relation V = kT^(2//3)...
Text Solution
|
- Three samples of the same gas A,B and C (gamma=3//2) have initially eq...
Text Solution
|
- Volume versus temperature graph of two moles of helium gas is as shown...
Text Solution
|
- In the P-V diagram shown in figure ABC is a semicircle. The work done ...
Text Solution
|
- Heat is supplied to a diatomic gas at constant pressure. The ratio o...
Text Solution
|
- A gas undergoes a change of state during which 100J of heat is supplie...
Text Solution
|
- N moles of an ideal diatomic gas are in a cylinder at temperature T. s...
Text Solution
|
- Three moles of an ideal gas (Cp=7/2R) at pressure, PA and temperature ...
Text Solution
|
- In a thermodynamic process pressure of a fixed mass of a gas is change...
Text Solution
|
- In an adiabatic change, the pressure and temperature of a monoatomic g...
Text Solution
|
- The internal energy of an ideal gas increases during an isothermal pro...
Text Solution
|