Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM AND ACIDS-BASES
VGS PUBLICATION-BRILLIANT|Exercise NUMERICAL PROBLEMS|71 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VGS PUBLICATION-BRILLIANT|Exercise Long Answer Questions|24 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CHEMICAL EQUILIBRIUM AND ACIDS-BASES-ADDITIONAL QUESTIONS & ANSWERS
- PCl(5),PCl(3) and Cl(2) are at equilibrium at 500K and having concentr...
Text Solution
|
- The value of DeltaG^(ϴ) for the phosphorylation of glucose in glycolys...
Text Solution
|
- What will be the conjugate bases of the following Bronsted acids: HF, ...
Text Solution
|
- Write the conjugate acids for the following Bronsted bases: NH(2)^(-),...
Text Solution
|
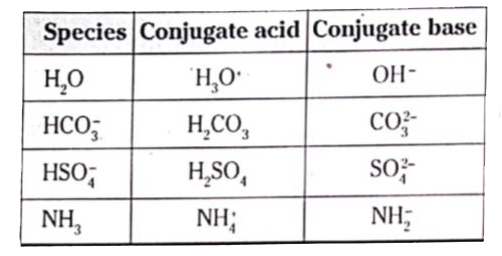
- The species: H(2)O,HCO(3)^(-),HSO(4)^(-) and NH(3) can act both as Bro...
Text Solution
|
- The concentration of hydrogen ion in a sample of soft drink is 3.8xx10...
Text Solution
|
- Calculate pH of a 1.0xx10^(-8) M solution of HCl.
Text Solution
|
- Calculate the solubility of A(2)X(3) is pure water, assuming that neit...
Text Solution
|