Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-ACIDS, BASES AND SALTS -Section-4
- Compounds such as alcohols and glucose contain hydrogen but are not ca...
Text Solution
|
- How do the acid rains influence the aquatic life ? What is our main re...
Text Solution
|
- Explain an activity to show the water of crystallisation in CuSO4.5H2O...
Text Solution
|
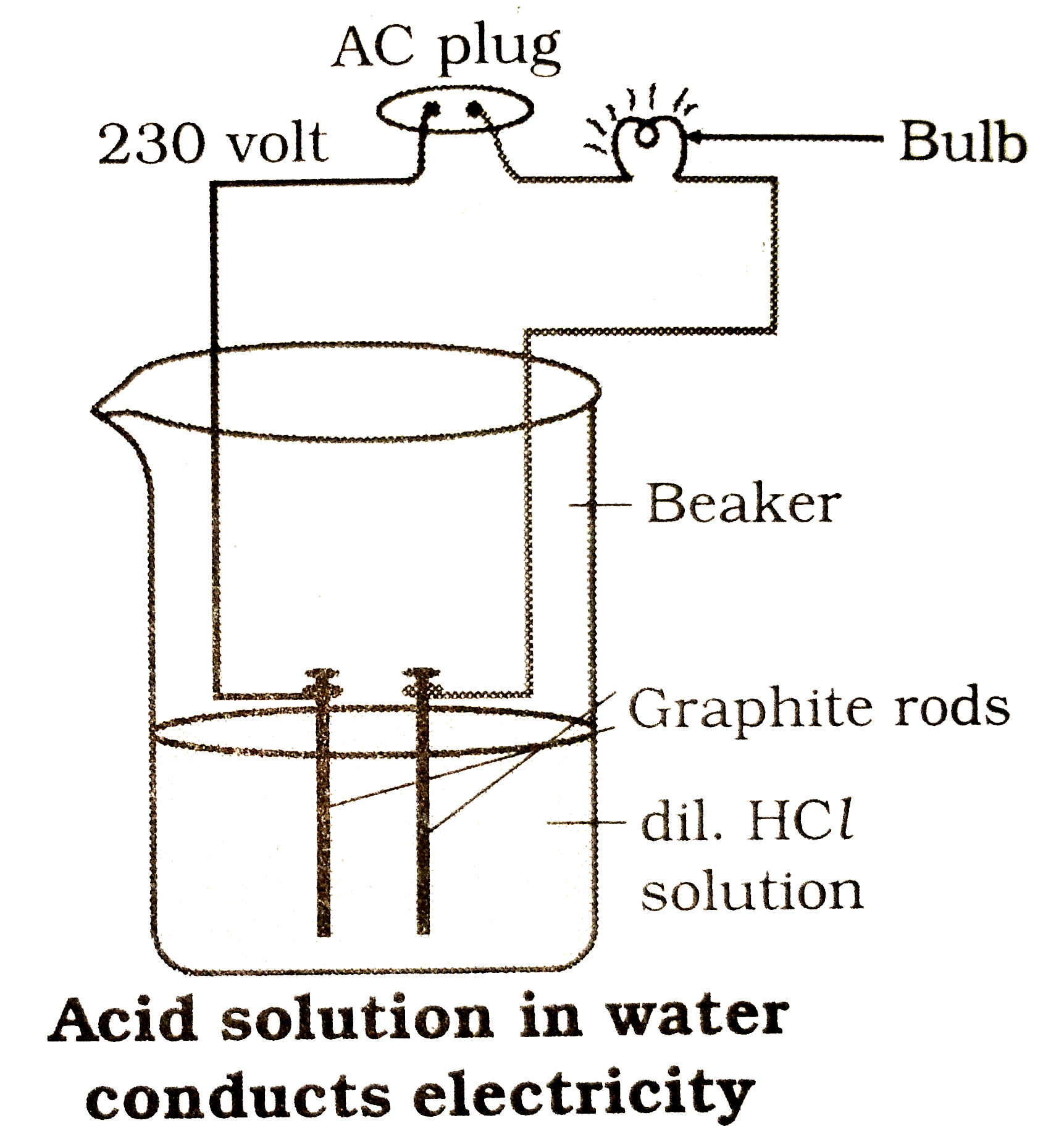
- Draw a neat diagram showing a base solution in water conducts electric...
Text Solution
|
- Read the information given in the table and answer the following quest...
Text Solution
|
- Read the information given in the table and answer the following quest...
Text Solution
|
- Read the information given in the table and answer the following quest...
Text Solution
|
- Read the information given in the table and answer the following quest...
Text Solution
|
- List out the materials required to test whether the solutions of given...
Text Solution
|
- Observe the following table and answer the questions given below. Th...
Text Solution
|
- Observe the following table and answer the questions given below. Th...
Text Solution
|
- Which of the above two produce maximum heat when they react ? What doe...
Text Solution
|
- Observe the following table and answer the questions given below. Th...
Text Solution
|
- List out the material for the experiment "when Hydrochloric acid react...
Text Solution
|
- Prepare a table based on the colour responses of acid, base and salt w...
Text Solution
|
- If the pH values of solutions X, Y and Z are 13, 6 and 2 respectively ...
Text Solution
|
- If the pH values of solutions X, Y and Z are 13, 6 and 2 respectively ...
Text Solution
|
- If the pH values of solutions X, Y and Z are 13, 6 and 2 respectively ...
Text Solution
|
- If the pH values of solutions X, Y and Z are 13, 6 and 2 respectively ...
Text Solution
|
- Describe how sodium hydroxide is obtained from common salt.
Text Solution
|
- Describe chlor - alkali process.
Text Solution
|