Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - IV 4 MARKS )|32 VideosSTRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - II 1 MARK)|71 VideosREFLECTION AND REFRACTION
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|24 VideosSUMMATIVE ASSESSMENT
VGS PUBLICATION-BRILLIANT|Exercise SUMMATIVE ASSESSMENT|28 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-STRUCTURE OF ATOM -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - III 2 MARKS QUESTIONS )
- Guess the orbital. If 1) It's energy lies in between the energies o...
Text Solution
|
- The electronic configuration of an atom is as follows 1s^(2) 2s^(2) 2...
Text Solution
|
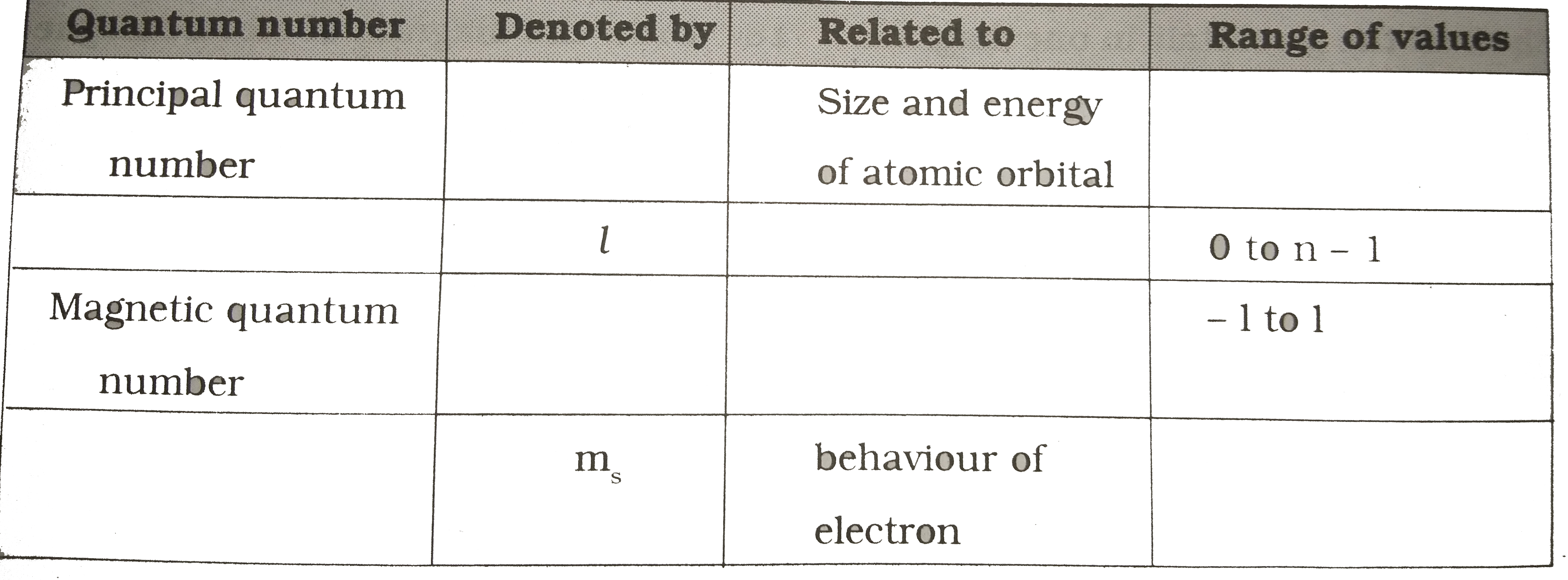
- Fill the following table and write the rule which you use in filling t...
Text Solution
|
- An electron is an atom has the following set four quantum numbers to w...
Text Solution
|
- You know that the distribution of electrons in shells, sub-shells and ...
Text Solution
|
- You know that the distribution of electrons in shells, sub-shells and ...
Text Solution
|
- The differentiating electron in one atom is 4p^(1) . Write the set ...
Text Solution
|
- The differentiating electron in one atom is 4p^(1) . Write the full ...
Text Solution
|
- For n = 3. Write all the m(l) values.
Text Solution
|
- For n = 3. Write all the sub-shells
Text Solution
|
- Write the name of the atom and electronic configuration for the given ...
Text Solution
|
- Where does the 6^(th) electron go?
Text Solution
|
- Among 3d, 4s, 4p orbitals, which one has least orbital energy ? Why...
Text Solution
|
- Among 3d, 4s, 4p orbitals, which one fills last by an electron? And...
Text Solution
|
- Show all the p-orbitals in one diagram.
Text Solution
|
- Draw d(x^(2) - y^(2)) orbital and P(z) orbital.
Text Solution
|
- Draw the diagram of electromagnetic wave
Text Solution
|
- How do you appreciate the Planck?
Text Solution
|
- What are the minimum and maximum frequency of a visible light ?
Text Solution
|
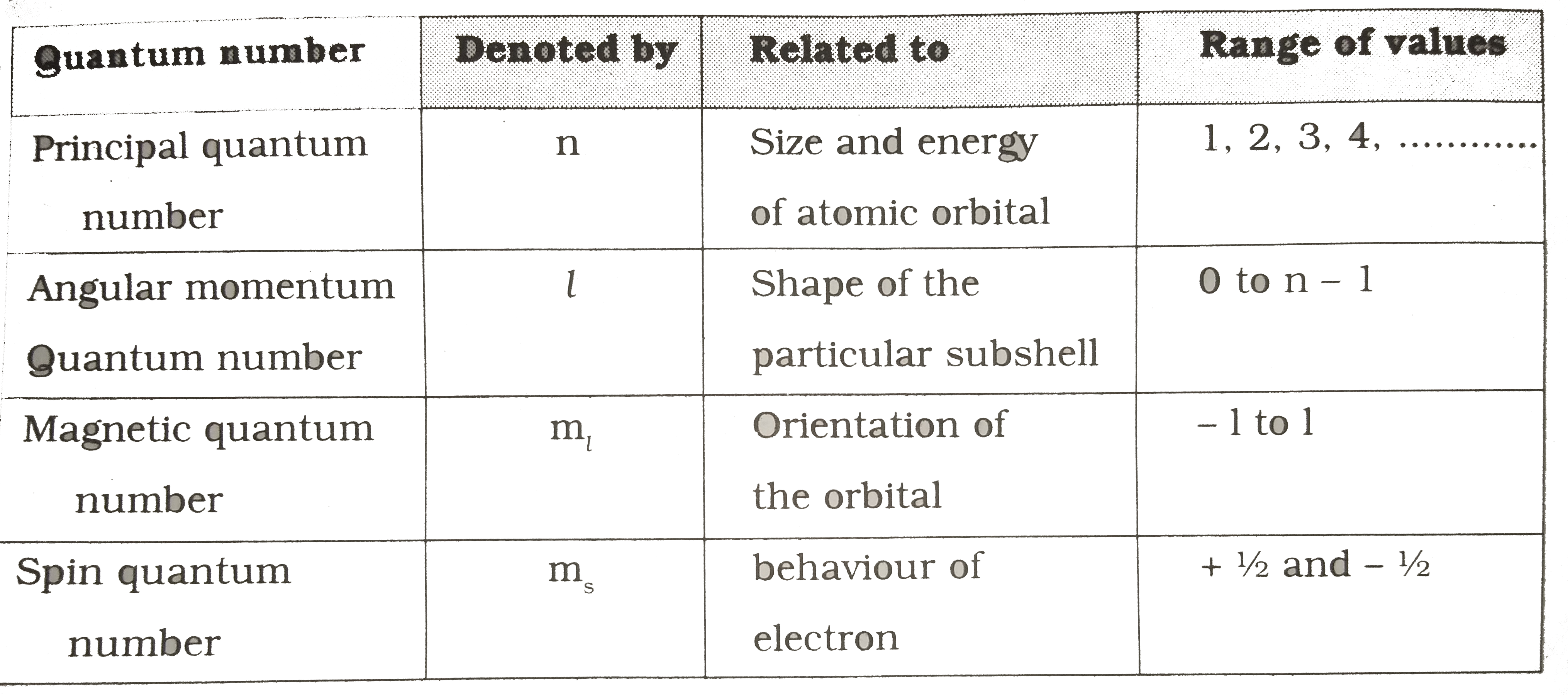
- Complete the following table based on quantum numbers related to atomi...
Text Solution
|