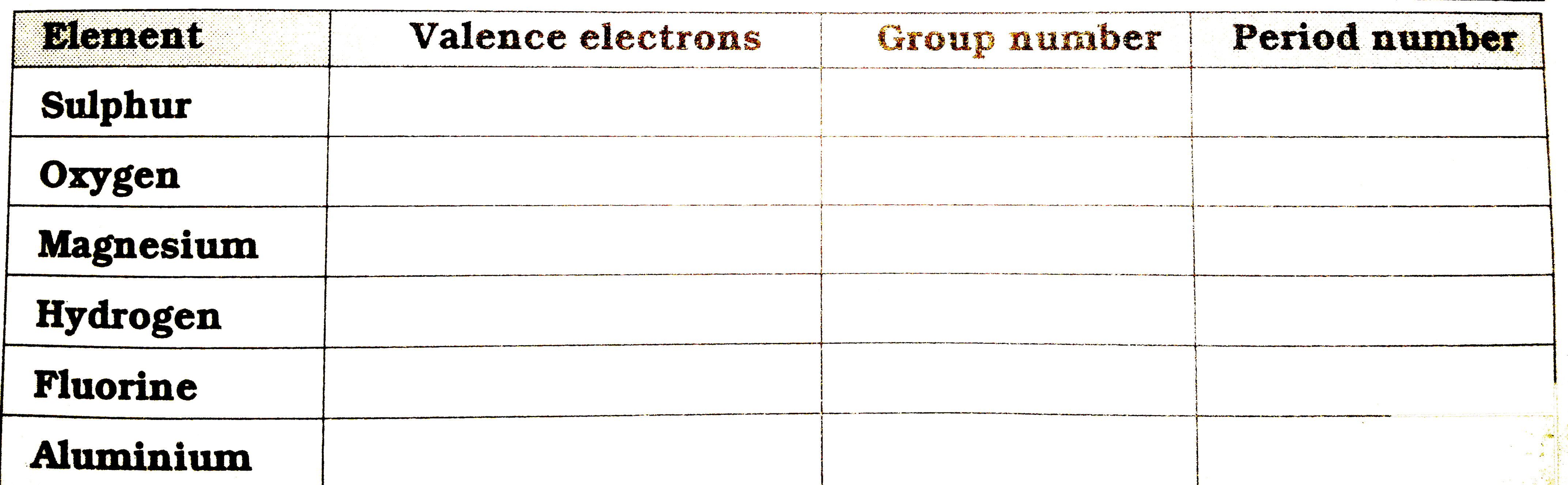
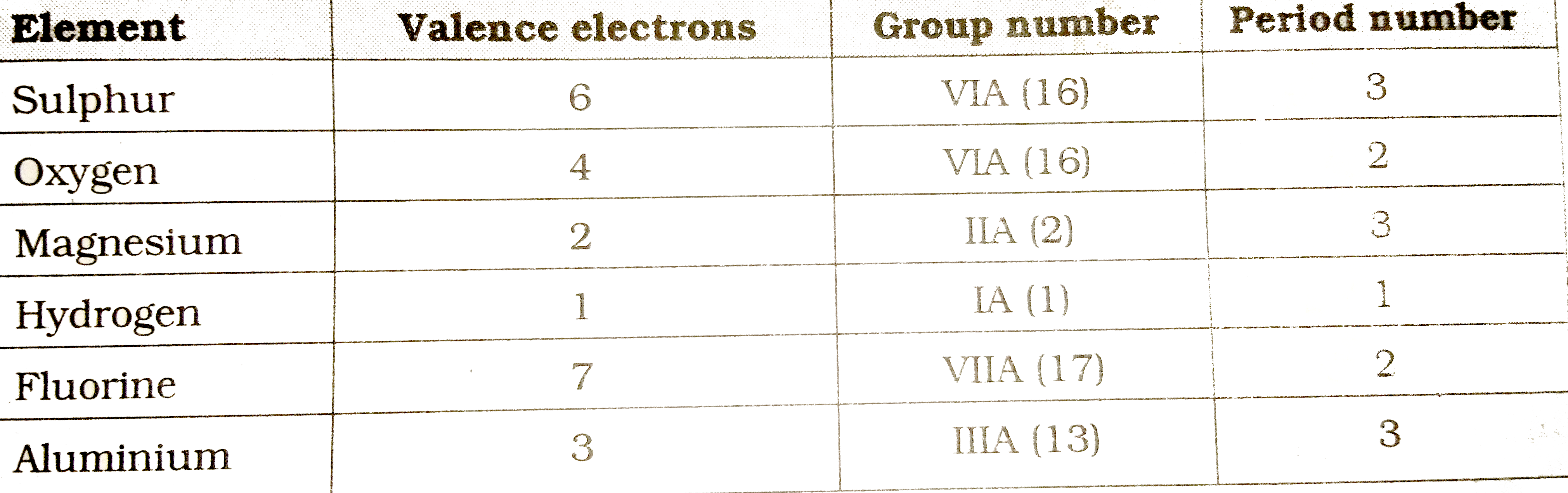
Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise ACTIVITIES|7 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise THINK AND DISCUSS ( 1 MARK QUESTIONS)|4 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise MULTIPLE CHOICE QUESTIONS|4 VideosCHEMICAL REACTIONS AND EQUATIONS
VGS PUBLICATION-BRILLIANT|Exercise OBJECTIVE TYPE QUESTIONS|80 VideosCLASSIFICATION OF ELEMENTS-THE PERIODIC TABLES
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|351 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE -TRY THESE
- Newlands proposed the law of octaves . Mendeleeff suggested eight ...
Text Solution
|
- State the number of valence electrons , the group number and ...
Text Solution
|
- State whether the following elements belong to a Group (G ) , Pe...
Text Solution
|
- S- block and p - block elements except 18 th group elements a...
Text Solution
|
- Identify the elements that has the lower ionization energy in e...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- In period 2 element X is to the right of element Y . Then , ...
Text Solution
|
- On the basis of atomic numbers predict to which block the ele...
Text Solution
|
- Aluminium does not react with water at room temperature but r...
Text Solution
|
- Collect the information about reactivity of VIIA group elements...
Text Solution
|
- How do you appreciate the role of electronic configuration of th...
Text Solution
|
- How do the positions of elements in the periodic table help you...
Text Solution
|