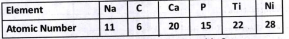
Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -III) (CONCEPTUAL UNDERSTANDING )|4 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -III) (ASKING QUESTIONS AND MAKING HYPOTHESIS )|7 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -II) (APPLICATION TO DAILY LIFE , CONCERN TO BIODIVERSITY )|3 VideosCHEMICAL REACTIONS AND EQUATIONS
VGS PUBLICATION-BRILLIANT|Exercise OBJECTIVE TYPE QUESTIONS|80 VideosCLASSIFICATION OF ELEMENTS-THE PERIODIC TABLES
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|351 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -III)
- An element has atomic number 17. Where would you expect this ...
Text Solution
|
- How do you appreciate the special nature of inert gases ?
Text Solution
|
- The Atomic number of an element is 35 where would you expect ...
Text Solution
|
- Why were Dobereiner , Newlands and Mendeleeff not 100 % successf...
Text Solution
|
- Observe the electronic condigurations given below and write th...
Text Solution
|
- Observe the electronic condigurations given below and write th...
Text Solution
|
- Observe the information provided in the table and answer the questions...
Text Solution
|
- Observe the information provided in the table and answer the ques...
Text Solution
|
- Imagine , which one in each of the following pairs is large in...
Text Solution
|
- Imagine , which one in each of the following pairs is large in...
Text Solution
|