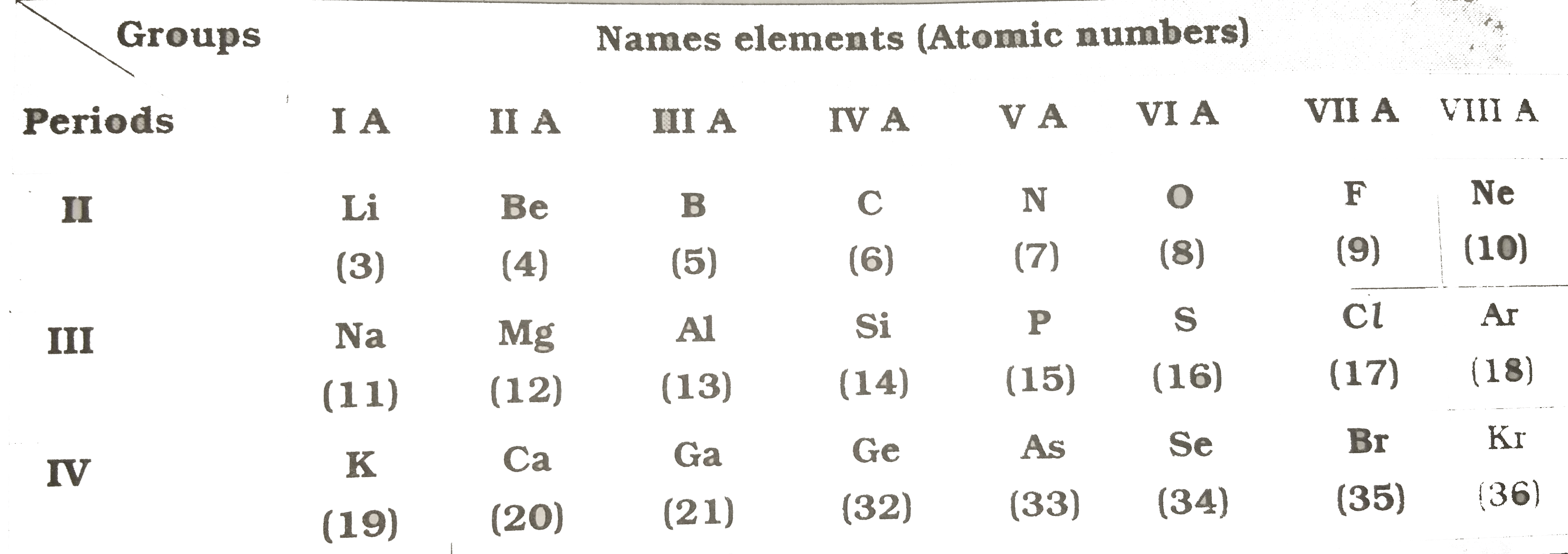
Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (ASKING QUESTIONS AND MAKING HYPOTHESIS )|3 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (INFORMATION SKILLS AND PROJECTS )|21 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -III) (APPLICATION TO DAILY LIFE , CONCERN TO BIODIVERSITY )|1 VideosCHEMICAL REACTIONS AND EQUATIONS
VGS PUBLICATION-BRILLIANT|Exercise OBJECTIVE TYPE QUESTIONS|80 VideosCLASSIFICATION OF ELEMENTS-THE PERIODIC TABLES
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|351 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV)
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- Two elements X and Y belong to Groups 1 and 2 respectively in th...
Text Solution
|
- In the table given below names of some elements of families are...
Text Solution
|
- What are the factors which influence electromagnetic energy '?
Text Solution
|
- How are the elements arranged into groups and periods in the mo...
Text Solution
|
- Answer the following from the above information . Which eleme...
Text Solution
|
- Answer the following from the above information . Mention tw...
Text Solution
|
- Answer the following from the above information . Name the ...
Text Solution
|
- Answer the following from the above information . Which ele...
Text Solution
|
- Observe the information and answer the following questions ...
Text Solution
|
- Observe the information and answer the following questions ...
Text Solution
|
- Observe the information and answer the following questions w...
Text Solution
|
- Observe the information and answer the following questions ...
Text Solution
|
- Explain the significance of three quantum numbers in predicting...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|