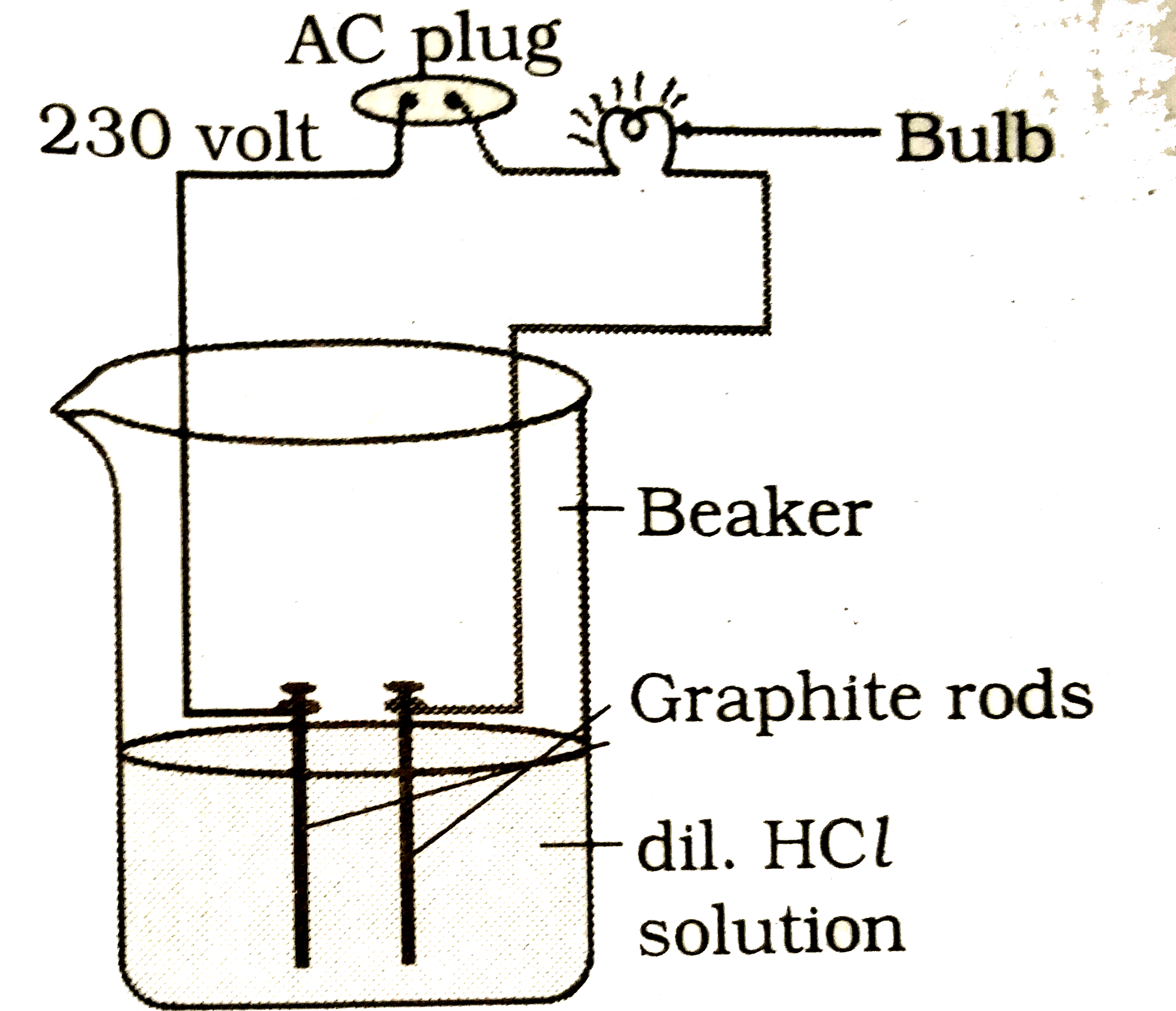
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-NEW MODEL PAPER CLASS X - PHYSICAL SCIENCE-SECTION - IV
- Write the differences between evaporation and boiling?
Text Solution
|
- Explain Faraday's law of induction with the help of an activity?
Text Solution
|
- The acidity of acids is attributed to the H^+. ions produced by thein ...
Text Solution
|
- Explain the significance of three quantum numbers in predicting the po...
Text Solution
|
- Write an experimental procedure to obtain the relation between angle o...
Text Solution
|
- Draw the experimental set-up to verify V//I is constant for a conducto...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|
- Answer the following question based on the values of the atomic...
Text Solution
|
- Complete the following table based on functional groups of organic com...
Text Solution
|
- Draw the ray diagrams for the following positions of objects in front ...
Text Solution
|
- Draw the ray diagrams for the following positions of objects in front ...
Text Solution
|
- Draw the neat diagram of froth floatation process for the concentratio...
Text Solution
|