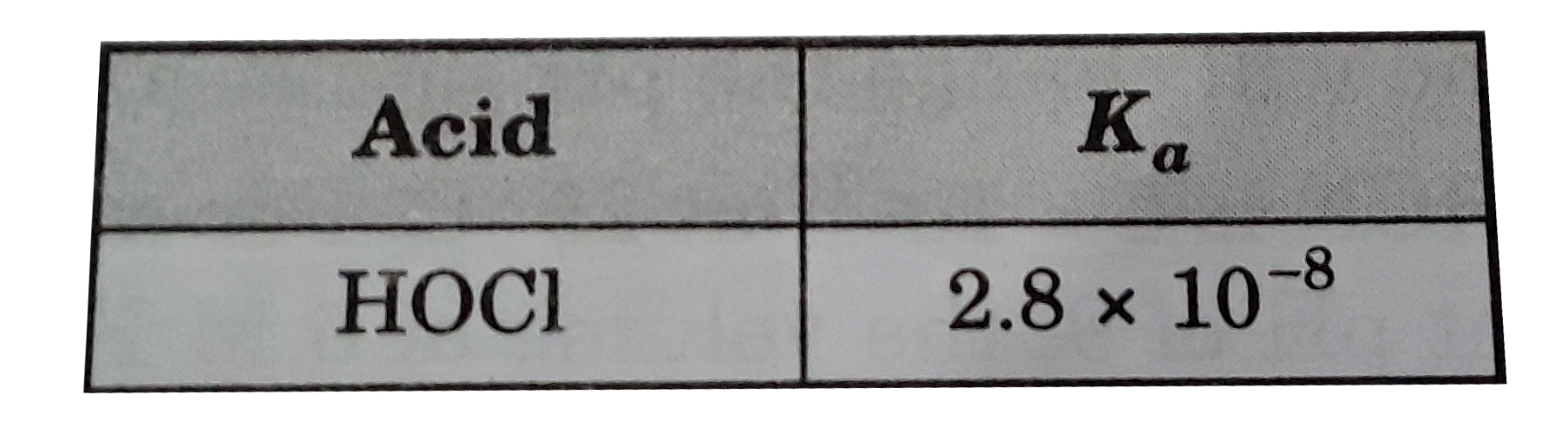
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-IONIC EQUILIBRIUM-All Questions
- A 0.010 M solution of a weak acid that produces one OH^- pe molecule o...
Text Solution
|
- What is the Kb of a weak base that produces one OH^- per molecule if a...
Text Solution
|
- What is the [OH^-] of a 0.65 M solution of NaOCl ?
Text Solution
|
- Acetylsalicylic acid (aspirin) behaves as an acid according to the equ...
Text Solution
|
- Calculate the [H^+] in a 0.25 M solution of methylamine, CH3NH2(Kb=4.4...
Text Solution
|
- What is the [H^+] in a solution in which [HA]=4.0xx10^(-2) and [A^-]=2...
Text Solution
|
- What is the [H^+] in a 0.10 N solution of ascorbic acid, C6H8O6?
Text Solution
|
- The degree of hydrolysis of 0.1 M solution of conjugate base of HA is ...
Text Solution
|
- The hydrolysis of an ester was carried out with 0.1 M H(2)SO(4) and 0....
Text Solution
|
- Which salt gives the most acidic 0.1 M solution in water ?
Text Solution
|
- A 0.1 M solution of which substances is most acidic ?
Text Solution
|
- What is the pH of a 0.15 M solution of hydrazine , N2H4?
Text Solution
|
- HOCl(aq)toH^(+)(aq)+OCl^(-)(aq) The ionization of hypochlorous acid ...
Text Solution
|
- The ionization of benzoic acid is represented by this equation C6H5C...
Text Solution
|
- pH when equal volume of 0.1 M HA (Ka=10^(-5)) and 1 M HB (Ka=10^(-6)) ...
Text Solution
|
- What is the [H^+] of a 0.075 M solution of the acid HA ?
Text Solution
|
- When a salt of weak acid and weak base is dissolved in water, the pH o...
Text Solution
|
- An aqueous solution of CH3COOH has a pH=3 and acid dissociation consta...
Text Solution
|
- What is the aq. Ammonia concentration of a solution prepared by dissol...
Text Solution
|
- 100 mL of 0.5 M hydrazoic acid (N3H, Ka=3.6xx10^(-4)) and 400 mL of 0....
Text Solution
|