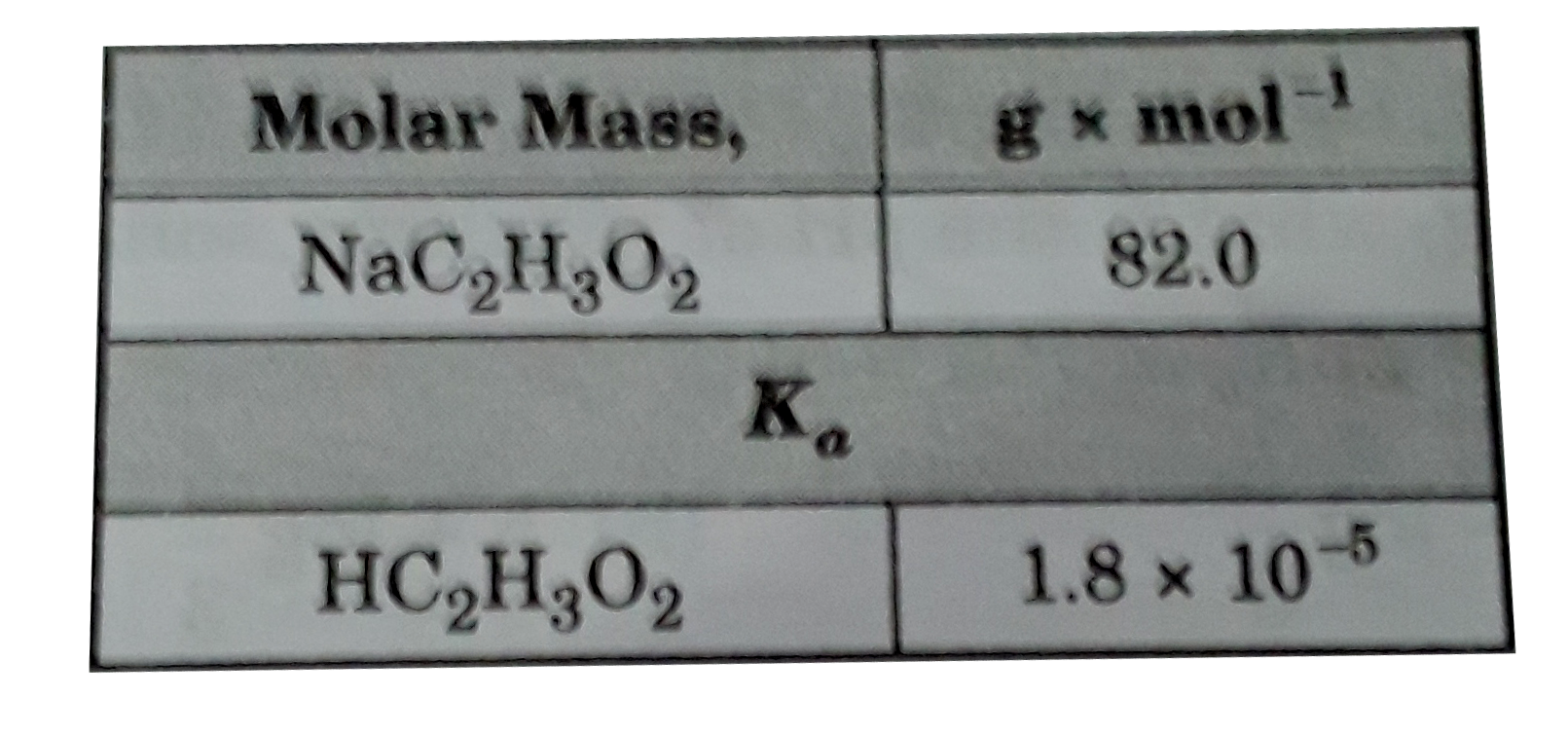A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-IONIC EQUILIBRIUM-All Questions
- A 75 mL solution that is 0.10 M in HC2H3O2 and 0.10 M in NaC2H3O2 has ...
Text Solution
|
- What happens when NH4Cl is added to solution of NH4OH ?
Text Solution
|
- What is the pH of a solution made by adding 0.41 g of NaC2H3O2 to 100 ...
Text Solution
|
- What will happen to the pH of a buffer solution when a small amount of...
Text Solution
|
- Which mixtures form buffer solution ? (P)100 mL of 0.200 M HF+200 mL...
Text Solution
|
- Which of these mixtures constitute buffer solutions ? Mixture 1 : 25...
Text Solution
|
- What happens to the pH of a buffer solution when it is diluted by a fa...
Text Solution
|
- What is [H3O^+] in a solution formed by dissolving 1.00 g NH4Cl (M=53....
Text Solution
|
- What volumes of 0.200 M HNO2 and 0.200 M NaNO2 are required to make 50...
Text Solution
|
- 100 mL solution (I) of buffer containin 0.1 (M) HA and 0.2 (M)A^-, is ...
Text Solution
|
- What is the pH of 1.00 L sample of a buffer solution containing 0.10 m...
Text Solution
|
- Which pair of solutions forms a buffer solution when equal volumes of ...
Text Solution
|
- A buffer solution made with NH3 and NH4Cl has a pH of 10.0 which proce...
Text Solution
|
- 1.0 L of an aqueous solution in which [H2CO3]=[HCO3^-]=0.10 M, has [H^...
Text Solution
|
- Which base is most suitable to prepare a buffer solution with a pH =11...
Text Solution
|
- HCOOH (aq) hArr H^+(aq) +HCOO^-(aq),Ka=1.7xx10^(-4) The ionization o...
Text Solution
|
- A student is asked to prepare a buffer solution with a pH of 4.00. Thi...
Text Solution
|
- What is the pH of a solution that is 0.20 M in HF and 0.40 M in NaF ? ...
Text Solution
|
- 1 M benzoic acid (pKa=4.20) and 1 M C6H5COONa solutions are given sepa...
Text Solution
|
- What is the difference in pH for 1//3 and 2//3 stages of neutralizatio...
Text Solution
|