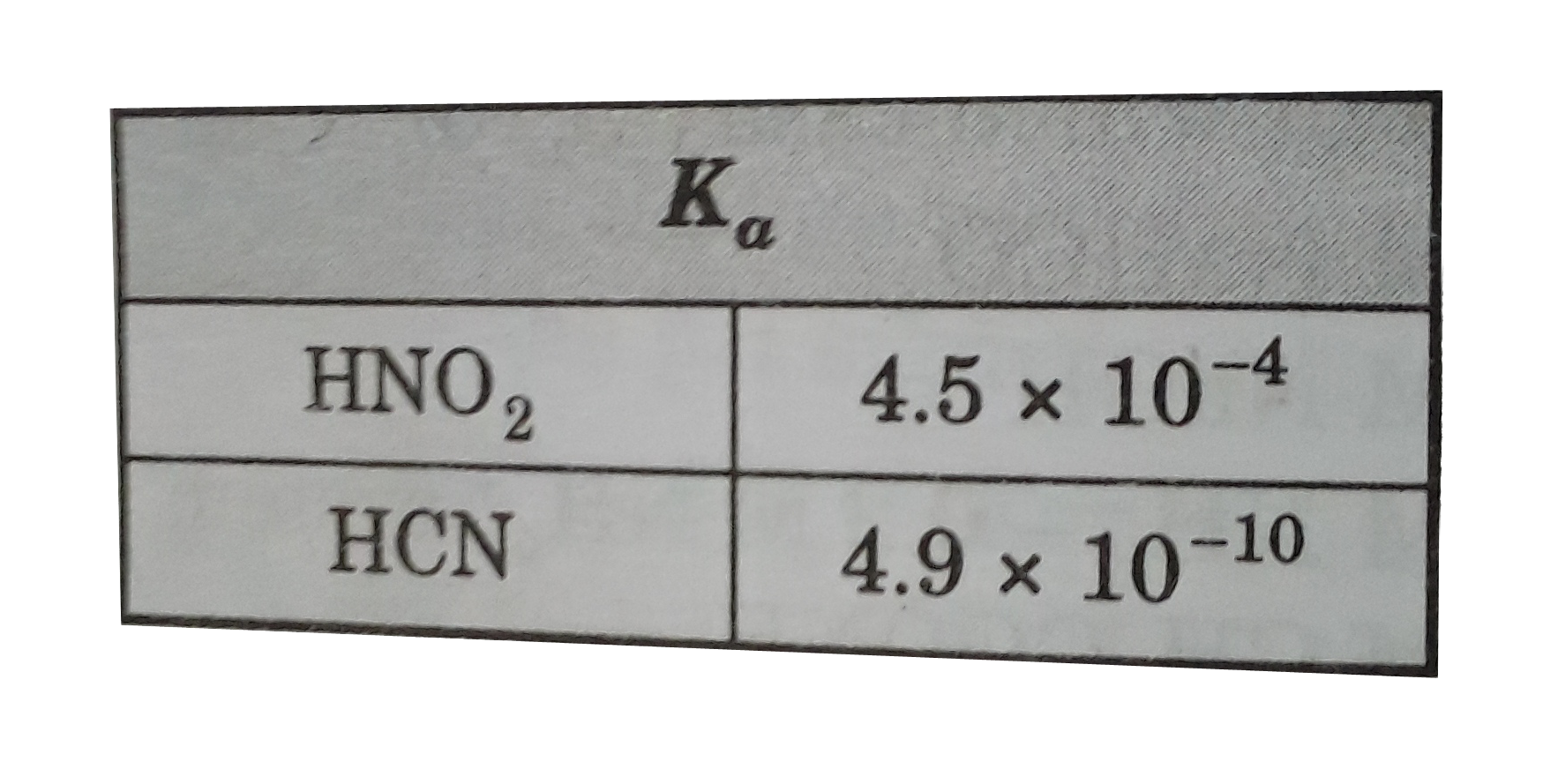
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-IONIC EQUILIBRIUM-All Questions
- Which base is most suitable to prepare a buffer solution with a pH =11...
Text Solution
|
- HCOOH (aq) hArr H^+(aq) +HCOO^-(aq),Ka=1.7xx10^(-4) The ionization o...
Text Solution
|
- A student is asked to prepare a buffer solution with a pH of 4.00. Thi...
Text Solution
|
- What is the pH of a solution that is 0.20 M in HF and 0.40 M in NaF ? ...
Text Solution
|
- 1 M benzoic acid (pKa=4.20) and 1 M C6H5COONa solutions are given sepa...
Text Solution
|
- What is the difference in pH for 1//3 and 2//3 stages of neutralizatio...
Text Solution
|
- To prepare a buffer of pH 8.26, amount of (NH(4))(2)SO(4) to be added ...
Text Solution
|
- How many moles of NaOCI must be added to 150 mL of 0.025 MHOCI to obta...
Text Solution
|
- 1 M NaCl and 1 M HCl are present in an aqueous solution. The solution ...
Text Solution
|
- The pK(a) of a weak acid (HA) is 4.5. The pOH of an aqueous buffered s...
Text Solution
|
- A buffer solution contains 1 mole of (NH(4))(2)SO(4) and 1 mole of NH(...
Text Solution
|
- In which of the following combinations, is buffer action expected ? ...
Text Solution
|
- Which solution is not a buffer solution ?
Text Solution
|
- Which species has the lowest concentration in a solution prepared mixi...
Text Solution
|
- Which may be added to one litre of water to act a buffer?
Text Solution
|
- 50 mL of 2N acetic acid mixed with 10 mL of 1N sodium acetate solution...
Text Solution
|
- The pH of an acidic buffer mixture is:
Text Solution
|
- A certain buffer solution contains equal concentartion of X^(Theta) an...
Text Solution
|
- pH of a mixture containing 0.10 M X^(-) and 0.20 M HX is: [pK(b)(X^(-)...
Text Solution
|
- The composition of an acidic buffer mixture made up of HA and NaA of t...
Text Solution
|