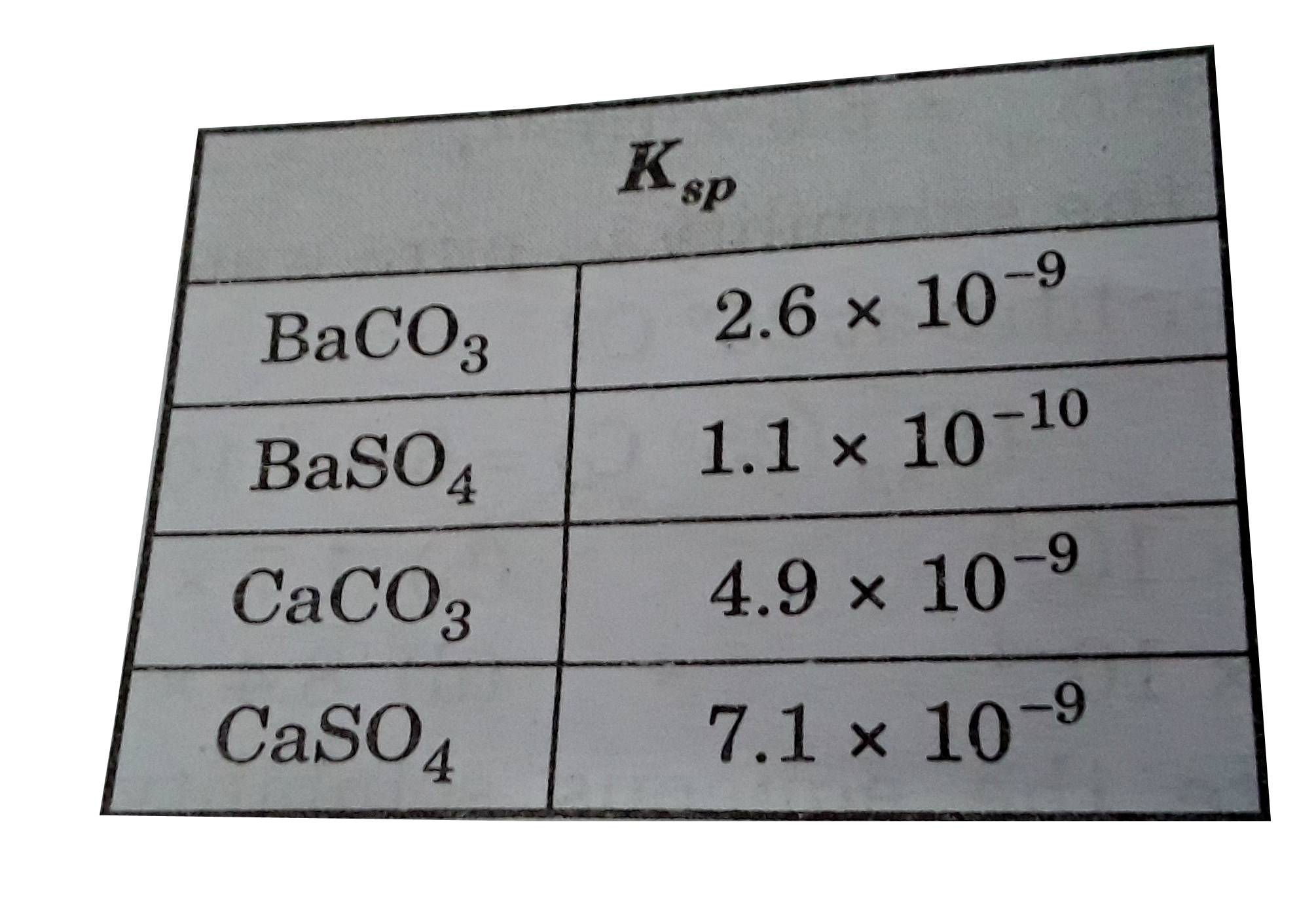
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-IONIC EQUILIBRIUM-All Questions
- For the reaction : PbI(2)(s)rarrPb^(2+)(aq)+2I^(-)(aq) K(sp)=8.4xx...
Text Solution
|
- The K(sp) of calcium flouride is 3.2xx10^(-11). Calculate the DeltaG^(...
Text Solution
|
- When the compounds below are arranged in order of increasing solubilit...
Text Solution
|
- When solid silver chloride (MM=143.4) is added to 100mL of H(2)O,1.9xx...
Text Solution
|
- A saturated solution of which compound has the lowest [Ca^(2+)]?
Text Solution
|
- What is the solubility of calcium hydroxide in mol.L^(-1)?
Text Solution
|
- If equal volumes of BaCI(2) and NaF solutions are mixed, which of thes...
Text Solution
|
- What is the solubility of magnesion carbonate, MgCO(3), in water at 25...
Text Solution
|
- What is the pH of a saturated solution of magnesium hydroxide, Mg(OH)(...
Text Solution
|
- Lead (II) fluoride (PbF(2)), lead(II) chloride (PbCl(2), lead(II) brom...
Text Solution
|
- How many moles of calcium fluoride , CaF(2), must be dissolved in 2.0 ...
Text Solution
|
- The solubility products (K(sp)) of three salts MX, MY(2) and MZ(3) are...
Text Solution
|
- A saturated solution of silver benzoate, C(6)H(5)CO(2)Ag has a pH of 8...
Text Solution
|
- A 500 mL saturated solution of MgCO(3) (M=84) is reduced to 120mL by e...
Text Solution
|
- The solubility of CaF(2) (K(sp)=3.4xx10^(-11)) in 0.01 M solution of N...
Text Solution
|
- The solubility product of BaCrO(4) is 2.4xx10^(-10)M^(2). The maximum ...
Text Solution
|
- What is the solubility of Al(OH)(3), (K(sp)=10^(-33)) in a buffer solu...
Text Solution
|
- The solubility of Fe(OH)(3) would be maximum in :
Text Solution
|
- The best explanation for the solubility of MnS in dil. HCI is that:
Text Solution
|
- Which of the following statements is correct for a solution saturated ...
Text Solution
|