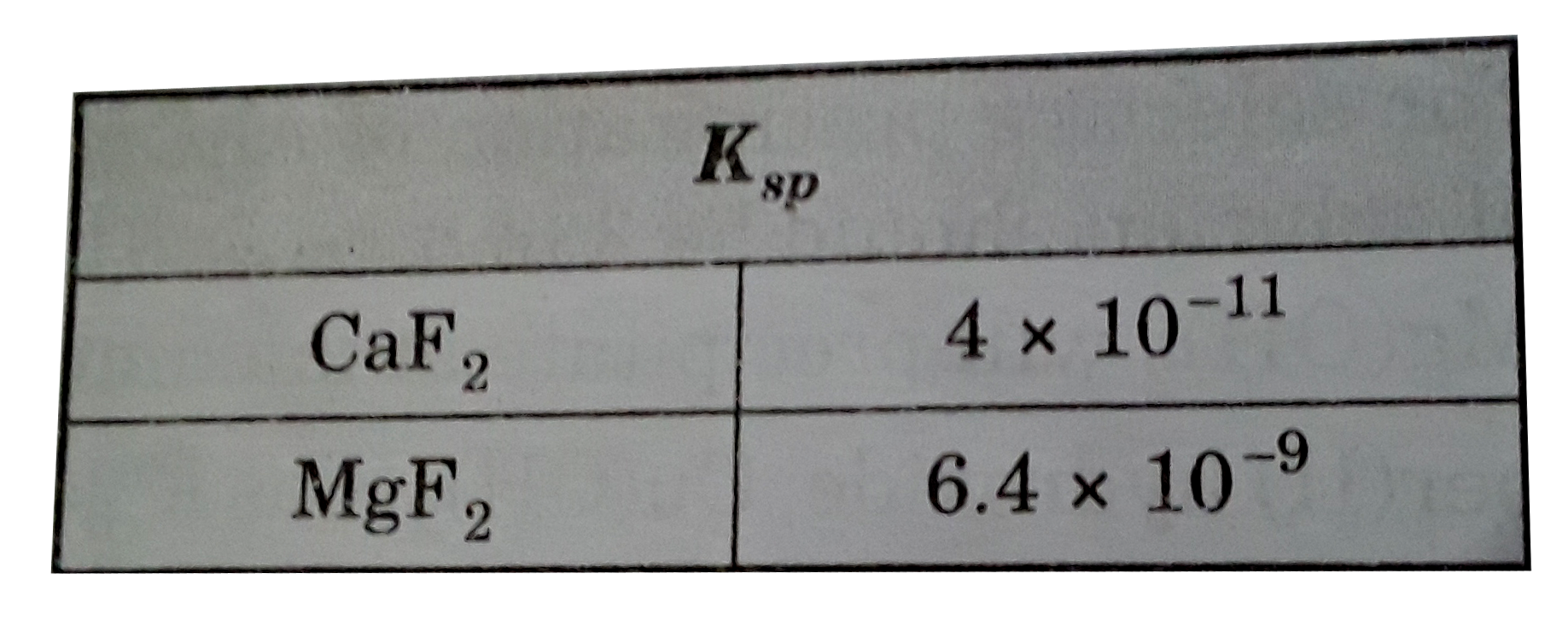
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-IONIC EQUILIBRIUM-All Questions
- The solubility of AgBrO(3) in aqueous solution depends on the presence...
Text Solution
|
- The pH of a saturated solution of Fe(OH)(2) is 8.67. What is the K(sp)...
Text Solution
|
- Which statement is correct about the initial precipitate that forms wh...
Text Solution
|
- Consider these mixtures : Which statement is correct?
Text Solution
|
- K(sp) of AgCl,Ag(2)CrO(4) and AgBr are 10^(-10),10^(-13) and 10^(-12) ...
Text Solution
|
- In which of the following solvents will AgBr has highest solubility?
Text Solution
|
- Solubility of Ag(2)CrO(4) (K(sp)=4xx10^(-13)) in 0.1 M K(2)CrO(4) sol...
Text Solution
|
- A solution containing 10^(-2) M MgCl(2) will just form precipitate whe...
Text Solution
|
- 1 mole of AgNO(3) is added to 10 litre of 1 M NH(3). What is the conce...
Text Solution
|
- What is the pH of a saturated solution of milk of magnesia, Mg(OH)(2) ...
Text Solution
|
- Solubility of BaF(2) in a solution of Ba(NO(3))(2), will be represente...
Text Solution
|
- Solubility of AgCl in pure water is 10^(-5) mol/litre at 25^(@)C then,...
Text Solution
|
- The solubility of Mg(OH)(2) in a buffer of pH=10 is found to be 0.0232...
Text Solution
|
- Assertion : The H(3)O^(+) has additional water molecules closely assoc...
Text Solution
|
- Assertion : The proton transfer reaction between NH(3) and H(2)O proce...
Text Solution
|
- Assertion : Aqueoous solutions of all strong acids contain only the sa...
Text Solution
|
- Assertion : Acids that have more than one proton that can be donated t...
Text Solution
|
- Assertion : 0.20 M solution of NaCN is more than basic than 0.20 M sol...
Text Solution
|
- Assertion : 0.20 M solution of NaCN is more than basic than 0.20 M sol...
Text Solution
|
- Assertion : Addition of HCl(aq) to HCOOH(aq) decreases the ionization ...
Text Solution
|